Stimulating GABAergic Neurons in the Nucleus Accumbens Core Alters the Trigeminal Neuropathic Pain Responses in a Rat Model of Infraorbital Nerve Injury
- PMID: 34445124
- PMCID: PMC8395143
- DOI: 10.3390/ijms22168421
Stimulating GABAergic Neurons in the Nucleus Accumbens Core Alters the Trigeminal Neuropathic Pain Responses in a Rat Model of Infraorbital Nerve Injury
Abstract
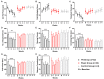
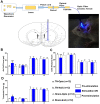
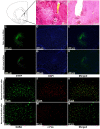
The nucleus accumbens core (NAcc) is an important component of brain reward circuitry, but studies have revealed its involvement in pain circuitry also. However, its effect on trigeminal neuralgia (TN) and the mechanism underlying it are yet to be fully understood. Therefore, this study aimed to examine the outcomes of optogenetic stimulation of NAcc GABAergic neurons in an animal model of TN. Animals were allocated into TN, sham, and control groups. TN was generated by infraorbital nerve constriction and the optogenetic virus was injected into the NAcc. In vivo extracellular recordings were acquired from the ventral posteromedial nucleus of the thalamus. Alterations of behavioral responses during stimulation "ON" and "OFF" conditions were evaluated. In vivo microdialysis was performed in the NAcc of TN and sham animals. During optogenetic stimulation, electrophysiological recordings revealed a reduction of both tonic and burst firing activity in TN animals, and significantly improved behavioral responses were observed as well. Microdialysis coupled with liquid chromatography/tandem mass spectrometry analysis revealed significant alterations in extracellular concentration levels of GABA, glutamate, acetylcholine, dopamine, and citrulline in NAcc upon optic stimulation. In fine, our results suggested that NAcc stimulation could modulate the transmission of trigeminal pain signals in the TN animal model.
Keywords: VPM thalamus; microdialysis; nucleus accumbens core; optogenetics; trigeminal neuralgia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

