Chitin-Derived AVR-48 Prevents Experimental Bronchopulmonary Dysplasia (BPD) and BPD-Associated Pulmonary Hypertension in Newborn Mice
- PMID: 34445253
- PMCID: PMC8395179
- DOI: 10.3390/ijms22168547
Chitin-Derived AVR-48 Prevents Experimental Bronchopulmonary Dysplasia (BPD) and BPD-Associated Pulmonary Hypertension in Newborn Mice
Abstract
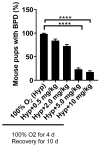
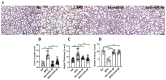
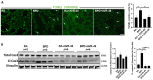
Bronchopulmonary dysplasia (BPD) is the most common complication of prematurity and a key contributor to the large health care burden associated with prematurity, longer hospital stays, higher hospital costs, and frequent re-hospitalizations of affected patients through the first year of life and increased resource utilization throughout childhood. This disease is associated with abnormal pulmonary function that may lead to BPD-associated pulmonary hypertension (PH), a major contributor to neonatal mortality and morbidity. In the absence of any definitive treatment options, this life-threatening disease is associated with high resource utilization during and after neonatal intensive care unit (NICU) stay. The goal of this study was to test the safety and efficacy of a small molecule derivative of chitin, AVR-48, as prophylactic therapy for preventing experimental BPD in a mouse model. Two doses of AVR-48 were delivered either intranasally (0.11 mg/kg), intraperitoneally (10 mg/kg), or intravenously (IV) (10 mg/kg) to newborn mouse pups on postnatal day (P)2 and P4. The outcomes were assessed by measuring total inflammatory cells in the broncho-alveolar lavage fluid (BALF), chord length, septal thickness, and radial alveolar counts of the alveoli, Fulton's Index (for PH), cell proliferation and cell death by immunostaining, and markers of inflammation by Western blotting and ELISA. The bioavailability and safety of the drug were assessed by pharmacokinetic and toxicity studies in both neonatal mice and rat pups (P3-P5). Following AVR-48 treatment, alveolar simplification was improved, as evident from chord length, septal thickness, and radial alveolar counts; total inflammatory cells were decreased in the BALF; Fulton's Index was decreased and lung inflammation and cell death were decreased, while angiogenesis and cell proliferation were increased. AVR-48 was found to be safe and the no-observed-adverse-effect level (NOAEL) in rat pups was determined to be 100 mg/kg when delivered via IV dosing with a 20-fold safety margin. With no reported toxicity and with a shorter half-life, AVR-48 is able to reverse the worsening cardiopulmonary phenotype of experimental BPD and BPD-PH, compared to controls, thus positioning it as a future drug candidate.
Keywords: AVR-48; BPD-associated pulmonary hypertension; bronchopulmonary dysplasia; chitohexaose; inflammation.
Conflict of interest statement
V.B. is on the Scientific Advisory Board of AyuVis Research Inc. for development of BPD drugs.
Figures







References
-
- Bhandari V. Bronchopulmonary Dysplasia. Springer; Berlin/Heidelberg, Germany: 2016.
-
- Lui K., Lee S.K., Kusuda S., Adams M., Vento M., Reichman B., Darlow B.A., Lehtonen L., Modi N., Norman M., et al. Trends in Outcomes for Neonates Born Very Preterm and Very Low Birth Weight in 11 High-Income Countries. J. Pediatr. 2019;215:32–40.e14. doi: 10.1016/j.jpeds.2019.08.020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

