Cathepsin L, a Target of Hypoxia-Inducible Factor-1-α, Is Involved in Melanosome Degradation in Melanocytes
- PMID: 34445307
- PMCID: PMC8395286
- DOI: 10.3390/ijms22168596
Cathepsin L, a Target of Hypoxia-Inducible Factor-1-α, Is Involved in Melanosome Degradation in Melanocytes
Abstract
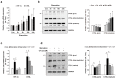
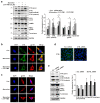
Hypoxic conditions induce the activation of hypoxia-inducible factor-1α (HIF-1α) to restore the supply of oxygen to tissues and cells. Activated HIF-1α translocates into the nucleus and binds to hypoxia response elements to promote the transcription of target genes. Cathepsin L (CTSL) is a lysosomal protease that degrades cellular proteins via the endolysosomal pathway. In this study, we attempted to determine if CTSL is a hypoxia responsive target gene of HIF-1α, and decipher its role in melanocytes in association with the autophagic pathway. The results of our luciferase reporter assay showed that the expression of CTSL is transcriptionally activated through the binding of HIF1-α at its promoter. Under autophagy-inducing starvation conditions, HIF-1α and CTSL expression is highly upregulated in melan-a cells. The mature form of CTSL is closely involved in melanosome degradation through lysosomal activity upon autophagosome-lysosome fusion. The inhibition of conversion of pro-CTSL to mature CTSL leads to the accumulation of gp100 and tyrosinase in addition to microtubule-associated protein 1 light chain 3 (LC3) II, due to decreased lysosomal activity in the autophagic pathway. In conclusion, we have identified that CTSL, a novel target of HIF-1α, participates in melanosome degradation in melanocytes through lysosomal activity during autophagosome-lysosome fusion.
Keywords: Cathepsin L; autophagy; hypoxia-inducible factor-1-alpha; melanosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

