Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage
- PMID: 34445319
- PMCID: PMC8395313
- DOI: 10.3390/ijms22168613
Membrane Association Modes of Natural Anticancer Peptides: Mechanistic Details on Helicity, Orientation, and Surface Coverage
Abstract
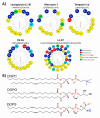
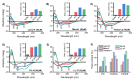
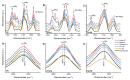
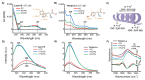
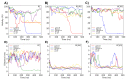
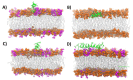
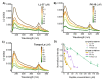
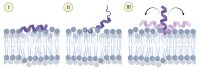
Anticancer peptides (ACPs) could potentially offer many advantages over other cancer therapies. ACPs often target cell membranes, where their surface mechanism is coupled to a conformational change into helical structures. However, details on their binding are still unclear, which would be crucial to reach progress in connecting structural aspects to ACP action and to therapeutic developments. Here we investigated natural helical ACPs, Lasioglossin LL-III, Macropin 1, Temporin-La, FK-16, and LL-37, on model liposomes, and also on extracellular vesicles (EVs), with an outer leaflet composition similar to cancer cells. The combined simulations and experiments identified three distinct binding modes to the membranes. Firstly, a highly helical structure, lying mainly on the membrane surface; secondly, a similar, yet only partially helical structure with disordered regions; and thirdly, a helical monomeric form with a non-inserted perpendicular orientation relative to the membrane surface. The latter allows large swings of the helix while the N-terminal is anchored to the headgroup region. These results indicate that subtle differences in sequence and charge can result in altered binding modes. The first two modes could be part of the well-known carpet model mechanism, whereas the newly identified third mode could be an intermediate state, existing prior to membrane insertion.
Keywords: anticancer peptides; flow-linear dichroism; molecular dynamics; peptide conformation; spectroscopy.
Conflict of interest statement
The authors declare that they have no conflict of interest. The authors also declare they have no financial or non-financial interests in any material discussed in this manuscript.
Figures

Similar articles
-
Model membrane interaction and DNA-binding of antimicrobial peptide Lasioglossin II derived from bee venom.Biochem Biophys Res Commun. 2013 Jan 4;430(1):1-6. doi: 10.1016/j.bbrc.2012.11.015. Epub 2012 Nov 14. Biochem Biophys Res Commun. 2013. PMID: 23159628
-
Transmembrane pores formed by human antimicrobial peptide LL-37.Biophys J. 2011 Apr 6;100(7):1688-96. doi: 10.1016/j.bpj.2011.02.018. Biophys J. 2011. PMID: 21463582 Free PMC article.
-
Lasioglossins: three novel antimicrobial peptides from the venom of the eusocial bee Lasioglossum laticeps (Hymenoptera: Halictidae).Chembiochem. 2009 Aug 17;10(12):2089-99. doi: 10.1002/cbic.200900133. Chembiochem. 2009. PMID: 19591185
-
Biomedical Relevance of Novel Anticancer Peptides in the Sensitive Treatment of Cancer.Biomolecules. 2021 Jul 29;11(8):1120. doi: 10.3390/biom11081120. Biomolecules. 2021. PMID: 34439786 Free PMC article. Review.
-
High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments.Biochim Biophys Acta. 2014 Sep;1838(9):2160-72. doi: 10.1016/j.bbamem.2014.01.016. Epub 2014 Jan 23. Biochim Biophys Acta. 2014. PMID: 24463069 Free PMC article. Review.
Cited by
-
Bioactive Earthworm Peptides Produced by Novel Protease-Producing Bacillus velezensis PM 35 and Its Bioactivities on Liver Cancer Cell Death via Apoptosis, Antioxidant Activity, Protection Against Oxidative Stress, and Immune Cell Activation.Front Microbiol. 2022 Aug 10;13:892945. doi: 10.3389/fmicb.2022.892945. eCollection 2022. Front Microbiol. 2022. PMID: 36033863 Free PMC article.
-
A Lipid-Sensitive Spider Peptide Toxin Exhibits Selective Anti-Leukemia Efficacy through Multimodal Mechanisms.Adv Sci (Weinh). 2024 Aug;11(32):e2404937. doi: 10.1002/advs.202404937. Epub 2024 Jul 4. Adv Sci (Weinh). 2024. PMID: 38962935 Free PMC article.
-
From oncolytic peptides to oncolytic polymers: A new paradigm for oncotherapy.Bioact Mater. 2023 Aug 14;31:206-230. doi: 10.1016/j.bioactmat.2023.08.007. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37637082 Free PMC article. Review.
-
Removal and identification of external protein corona members from RBC-derived extracellular vesicles by surface manipulating antimicrobial peptides.J Extracell Biol. 2023 Mar 8;2(3):e78. doi: 10.1002/jex2.78. eCollection 2023 Mar. J Extracell Biol. 2023. PMID: 38938416 Free PMC article.
-
Renovation as innovation: Repurposing human antibacterial peptide LL-37 for cancer therapy.Front Pharmacol. 2022 Aug 23;13:944147. doi: 10.3389/fphar.2022.944147. eCollection 2022. Front Pharmacol. 2022. PMID: 36081952 Free PMC article. Review.
References
-
- Gabernet G., Müller A.T., Hiss J.A., Schneider G. Membranolytic anticancer peptides. MedChemComm. 2016;7:2232–2245. doi: 10.1039/C6MD00376A. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

