From Menopause to Neurodegeneration-Molecular Basis and Potential Therapy
- PMID: 34445359
- PMCID: PMC8395405
- DOI: 10.3390/ijms22168654
From Menopause to Neurodegeneration-Molecular Basis and Potential Therapy
Abstract
The impacts of menopause on neurodegenerative diseases, especially the changes in steroid hormones, have been well described in cell models, animal models, and humans. However, the therapeutic effects of hormone replacement therapy on postmenopausal women with neurodegenerative diseases remain controversial. The steroid hormones, steroid hormone receptors, and downstream signal pathways in the brain change with aging and contribute to disease progression. Estrogen and progesterone are two steroid hormones which decline in circulation and the brain during menopause. Insulin-like growth factor 1 (IGF-1), which plays an import role in neuroprotection, is rapidly decreased in serum after menopause. Here, we summarize the actions of estrogen, progesterone, and IGF-1 and their signaling pathways in the brain. Since the incidence of Alzheimer's disease (AD) is higher in women than in men, the associations of steroid hormone changes and AD are emphasized. The signaling pathways and cellular mechanisms for how steroid hormones and IGF-1 provide neuroprotection are also addressed. Finally, the molecular mechanisms of potential estrogen modulation on N-methyl-d-aspartic acid receptors (NMDARs) are also addressed. We provide the viewpoint of why hormone therapy has inconclusive results based on signaling pathways considering their complex response to aging and hormone treatments. Nonetheless, while diagnosable AD may not be treatable by hormone therapy, its preceding stage of mild cognitive impairment may very well be treatable by hormone therapy.
Keywords: Alzheimer’s disease; IGF-1; NMDAR; estrogen; menopause; neurodegenerative disease.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors were not involved in the design of the study; the collection, analysis, and interpretation of the data; the writing of the report; and the decision to submit the article for publication.
Figures
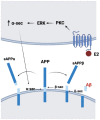
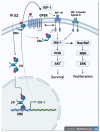
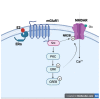
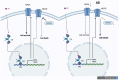
References
-
- Garre-Olmo J. Epidemiology of Alzheimer’s disease and other dementias. Rev. Neurol. 2018;66:377–386. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

