Altered Follicular Fluid Metabolic Pattern Correlates with Female Infertility and Outcome Measures of In Vitro Fertilization
- PMID: 34445441
- PMCID: PMC8395780
- DOI: 10.3390/ijms22168735
Altered Follicular Fluid Metabolic Pattern Correlates with Female Infertility and Outcome Measures of In Vitro Fertilization
Abstract
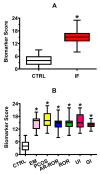
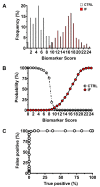
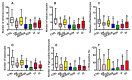
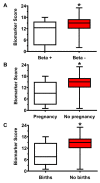
Nearly 40-50% of infertility problems are estimated to be of female origin. Previous studies dedicated to the analysis of metabolites in follicular fluid (FF) produced contrasting results, although some valuable indexes capable to discriminate control groups (CTRL) from infertile females (IF) and correlate with outcome measures of assisted reproduction techniques were in some instances found. In this study, we analyzed in blind FF of 35 control subjects (CTRL = patients in which inability to obtain pregnancy was exclusively due to a male factor) and 145 IF (affected by: endometriosis, n = 19; polycystic ovary syndrome, n = 14; age-related reduced ovarian reserve, n = 58; reduced ovarian reserve, n = 29; unexplained infertility, n = 14; genetic infertility, n = 11) to determine concentrations of 55 water- and fat-soluble low molecular weight compounds (antioxidants, oxidative/nitrosative stress-related compounds, purines, pyrimidines, energy-related metabolites, and amino acids). Results evidenced that 27/55 of them had significantly different values in IF with respect to those measured in CTRL. The metabolic pattern of these potential biomarkers of infertility was cumulated (in both CTRL and IF) into a Biomarker Score index (incorporating the metabolic anomalies of FF), that fully discriminated CTRL (mean Biomarker Score value = 4.00 ± 2.30) from IF (mean Biomarker Score value = 14.88 ± 3.09, p < 0.001). The Biomarker Score values were significantly higher than those of CTRL in each of the six subgroups of IF. Posterior probability curves and ROC curve indicated that values of the Biomarker Score clustered CTRL and IF into two distinct groups, based on the individual FF metabolic profile. Furthermore, Biomarker Score values correlated with outcome measures of ovarian stimulation, in vitro fertilization, number and quality of blastocysts, clinical pregnancy, and healthy offspring. These results strongly suggest that the biochemical quality of FF deeply influences not only the effectiveness of IVF procedures but also the following embryonic development up to healthy newborns. The targeted metabolomic analysis of FF (using empowered Redox Energy Test) and the subsequent calculation of the Biomarker Score evidenced a set of 27 low molecular weight infertility biomarkers potentially useful in the laboratory managing of female infertility and to predict the success of assisted reproduction techniques.
Keywords: amino acids; antioxidants; assisted reproduction techniques; biomarkers; energy metabolites; female infertility; follicular fluid; oxidative/nitrosative stress; targeted metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zhao H., Zhao Y., Li T., Li M., Li J., Li R., Liu P., Yu Y., Qiao J. Metabolism alteration in follicular niche: The nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015;86:295–307. doi: 10.1016/j.freeradbiomed.2015.05.013. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

