Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia
- PMID: 34445510
- PMCID: PMC8396240
- DOI: 10.3390/ijms22168800
Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia
Abstract
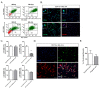
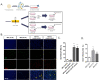
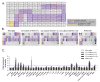
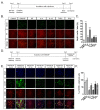
Microglia are resident immune cells of the central nervous system that act as brain-specific macrophages and are also known to regulate the innate immune functions of astrocytes through secretory molecules. This communication plays an important role in brain functions and homeostasis as well as in neuropathologic disease. In this study, we aimed to elucidate whether astrocytes and microglia could crosstalk to induce microglial polarization and proliferation, which can be further regulated under a microenvironment mimicking that of brain stroke. Microglia in a mixed glial culture showed increased survival and proliferation and were altered to M2 microglia; CD11b-GFAP+ astrocytes resulted in an approximately tenfold increase in microglial cell proliferation after the reconstitution of astrocytes. Furthermore, GM-CSF stimulated microglial proliferation approximately tenfold and induced them to become CCR7+ M1 microglia, which have a phenotype that could be suppressed by anti-inflammatory cytokines such as IL-4, IL-10, and substance P. In addition, the astrocytes in the microglial co-culture showed an A2 phenotype; they could be activated to A1 astrocytes by TNF-α and IFN-γ under the stroke-mimicking condition. Altogether, astrocytes in the mixed glial culture stimulated the proliferation of the microglia and M2 polarization, possibly through the acquisition of the A2 phenotype; both could be converted to M1 microglia and A1 astrocytes under the inflammatory stroke-mimicking environment. This study demonstrated that microglia and astrocytes could be polarized to M2 microglia and A2 astrocytes, respectively, through crosstalk in vitro and provides a system with which to explore how microglia and astrocytes may behave in the inflammatory disease milieu after in vivo transplantation.
Keywords: M1/M2 polarization; astrocyte; microglia; microglia and astrocyte crosstalk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

