Functional Hyaluronic Acid-Polylactic Acid/Silver Nanoparticles Core-Sheath Nanofiber Membranes for Prevention of Post-Operative Tendon Adhesion
- PMID: 34445516
- PMCID: PMC8396318
- DOI: 10.3390/ijms22168781
Functional Hyaluronic Acid-Polylactic Acid/Silver Nanoparticles Core-Sheath Nanofiber Membranes for Prevention of Post-Operative Tendon Adhesion
Abstract
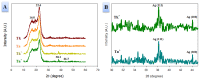
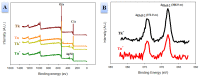
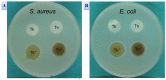
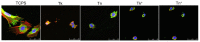
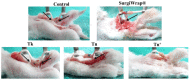
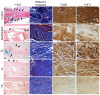
In this study, we prepared core-sheath nanofiber membranes (CSNFMs) with silver nanoparticles (Ag NPs) embedding in the polylactic acid (PLA) nanofiber sheath and hyaluronic acid (HA) in the nanofiber core. The PLA/Ag NPs sheath provides mechanical support as well as anti-bacterial and anti-inflammatory properties. The controlled release of HA from the core could exert anti-adhesion effects to promote tendon sliding while reducing fibroblast attachment. From the microfibrous structural nature of CSNFMs, they function as barrier membranes to reduce fibroblast penetration without hampering nutrient transports to prevent post-operative peritendinous adhesion. As the anti-adhesion efficacy will depend on release rate of HA from the core as well as Ag NP from the sheath, we fabricated CSNFMs of comparable fiber diameter, but with thick (Tk) or thin (Tn) sheath. Similar CSNFMs with thick (Tk+) and thin (Tn+) sheath but with embedded Ag NPs in the sheath were also prepared. The physico-chemical properties of the barrier membranes were characterized in details, together with their biological response including cell penetration, cell attachment and proliferation, and cytotoxicity. Peritendinous anti-adhesion models in rabbits were used to test the efficacy of CSNFMs as anti-adhesion barriers, from gross observation, histology, and biomechanical tests. Overall, the CSNFM with thin-sheath and Ag NPs (Tn+) shows antibacterial activity with low cytotoxicity, prevents fibroblast penetration, and exerts the highest efficacy in reducing fibroblast attachment in vitro. From in vivo studies, the Tn+ membrane also shows significant improvement in preventing peritendinous adhesions as well as anti-inflammatory efficacy, compared with Tk and Tn CSNFMs and a commercial adhesion barrier film (SurgiWrap®) made from PLA.
Keywords: anti-adhesion; core-sheath nanofibers; electrospinning; hyaluronic acid; polylactic acid; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









References
-
- de Melo F., Carrijo A., Hong K., Trumbic B., Vercesi F., Waldorf H.A., Zenker S. Minimally Invasive Aesthetic Treatment of the Face and Neck Using Combinations of a PCL-Based Collagen Stimulator, PLLA/PLGA Suspension Sutures, and Cross-Linked Hyaluronic Acid. Clin. Cosmet. Investig. Dermatol. 2020;13:333–344. doi: 10.2147/CCID.S248280. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

