Radiotherapy of High-Grade Gliomas: First Half of 2021 Update with Special Reference to Radiosensitization Studies
- PMID: 34445646
- PMCID: PMC8396323
- DOI: 10.3390/ijms22168942
Radiotherapy of High-Grade Gliomas: First Half of 2021 Update with Special Reference to Radiosensitization Studies
Abstract
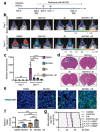
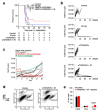
Albeit the effort to develop targeted therapies for patients with high-grade gliomas (WHO grades III and IV) is evidenced by hundreds of current clinical trials, radiation remains one of the few effective therapeutic options for them. This review article analyzes the updates on the topic "radiotherapy of high-grade gliomas" during the period 1 January 2021-30 June 2021. The high number of articles retrieved in PubMed using the search terms ("gliom* and radio*") and manually selected for relevance indicates the feverish research currently ongoing on the subject. During the last semester, significant advances were provided in both the preclinical and clinical settings concerning the diagnosis and prognosis of high-grade gliomas, their radioresistance, and the inevitable side effects of their treatment with radiation. The novel information concerning tumor radiosensitization was of special interest in terms of therapeutic perspective and was discussed in detail.
Keywords: high-grade-glioma; radiotherapy; sensitization; update.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

