HPE1, an Effector from Zebra Chip Pathogen Interacts with Tomato Proteins and Perturbs Ubiquitinated Protein Accumulation
- PMID: 34445707
- PMCID: PMC8396652
- DOI: 10.3390/ijms22169003
HPE1, an Effector from Zebra Chip Pathogen Interacts with Tomato Proteins and Perturbs Ubiquitinated Protein Accumulation
Abstract
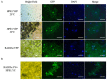
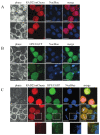
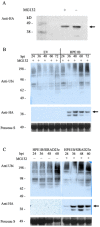
The gram-negative bacterial genus Liberibacter includes economically important pathogens, such as 'Candidatus Liberibacter asiaticus' that cause citrus greening disease (or Huanglongbing, HLB) and 'Ca. Liberibacter solanacearum' (Lso) that cause zebra chip disease in potato. Liberibacter pathogens are fastidious bacteria transmitted by psyllids. Pathogen manipulation of the host' and vector's immune system for successful colonization is hypothesized to be achieved by Sec translocon-dependent effectors (SDE). In previous work, we identified hypothetical protein effector 1 (HPE1), an SDE from Lso, that acts as a suppressor of the plant's effector-triggered immunity (ETI)-like response. In this study, using a yeast two-hybrid system, we identify binding interactions between tomato RAD23 proteins and HPE1. We further show that HPE1 interacts with RAD23 in both nuclear and cytoplasmic compartments in planta. Immunoblot assays show that HPE1 is not ubiquitinated in the plant cell, but rather the expression of HPE1 induced the accumulation of other ubiquitinated proteins. A similar accumulation of ubiquitinated proteins is also observed in Lso infected tomato plants. Finally, earlier colonization and symptom development following Lso haplotype B infection are observed in HPE1 overexpressing plants compared to wild-type plants. Overall, our results suggest that HPE1 plays a role in virulence in Lso pathogenesis, possibly by perturbing the ubiquitin-proteasome system via direct interaction with the ubiquitin-like domain of RAD23 proteins.
Keywords: Ca. Liberibacter solanacearum; HPE1; RAD23; effector; ubiquitin-proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
CLIBASIA_00460 Disrupts Hypersensitive Response and Interacts with Citrus Rad23 Proteins.Int J Mol Sci. 2022 Jul 16;23(14):7846. doi: 10.3390/ijms23147846. Int J Mol Sci. 2022. PMID: 35887193 Free PMC article.
-
Lso-HPE1, an Effector of 'Candidatus Liberibacter solanacearum', Can Repress Plant Immune Response.Phytopathology. 2020 Mar;110(3):648-655. doi: 10.1094/PHYTO-07-19-0252-R. Epub 2020 Jan 10. Phytopathology. 2020. PMID: 31697198
-
Effects of 'Candidatus Liberibacter solanacearum' haplotypes A and B on tomato gene expression and geotropism.BMC Plant Biol. 2022 Mar 30;22(1):156. doi: 10.1186/s12870-022-03505-z. BMC Plant Biol. 2022. PMID: 35354405 Free PMC article.
-
The Candidatus Liberibacter-Host Interface: Insights into Pathogenesis Mechanisms and Disease Control.Annu Rev Phytopathol. 2017 Aug 4;55:451-482. doi: 10.1146/annurev-phyto-080516-035513. Epub 2017 Jun 21. Annu Rev Phytopathol. 2017. PMID: 28637377 Review.
-
Interactions of Liberibacter Species with Their Psyllid Vectors: Molecular, Biological and Behavioural Mechanisms.Int J Mol Sci. 2022 Apr 5;23(7):4029. doi: 10.3390/ijms23074029. Int J Mol Sci. 2022. PMID: 35409386 Free PMC article. Review.
Cited by
-
'Candidatus liberibacter solanacearum' protein CKC_05770 interacts in vivo with tomato APX6 and APX7.Sci Rep. 2025 Mar 28;15(1):10826. doi: 10.1038/s41598-025-93367-w. Sci Rep. 2025. PMID: 40155471 Free PMC article.
-
CLIBASIA_00460 Disrupts Hypersensitive Response and Interacts with Citrus Rad23 Proteins.Int J Mol Sci. 2022 Jul 16;23(14):7846. doi: 10.3390/ijms23147846. Int J Mol Sci. 2022. PMID: 35887193 Free PMC article.
-
Comparative genomics of the Liberibacter genus reveals widespread diversity in genomic content and positive selection history.Front Microbiol. 2023 Jun 26;14:1206094. doi: 10.3389/fmicb.2023.1206094. eCollection 2023. Front Microbiol. 2023. PMID: 37434713 Free PMC article.
References
-
- Munyaneza J.E. Zebra chip disease, Candidatus Liberibacter, and potato psyllid: A global threat to the potato industry. Am. J. Potato Res. 2015;92:230–235. doi: 10.1007/s12230-015-9448-6. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

