Multistep Signaling in Nature: A Close-Up of Geobacter Chemotaxis Sensing
- PMID: 34445739
- PMCID: PMC8396549
- DOI: 10.3390/ijms22169034
Multistep Signaling in Nature: A Close-Up of Geobacter Chemotaxis Sensing
Abstract
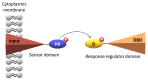
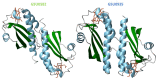
Environmental changes trigger the continuous adaptation of bacteria to ensure their survival. This is possible through a variety of signal transduction pathways involving chemoreceptors known as methyl-accepting chemotaxis proteins (MCP) that allow the microorganisms to redirect their mobility towards favorable environments. MCP are two-component regulatory (or signal transduction) systems (TCS) formed by a sensor and a response regulator domain. These domains synchronize transient protein phosphorylation and dephosphorylation events to convert the stimuli into an appropriate cellular response. In this review, the variability of TCS domains and the most common signaling mechanisms are highlighted. This is followed by the description of the overall cellular topology, classification and mechanisms of MCP. Finally, the structural and functional properties of a new family of MCP found in Geobacter sulfurreducens are revisited. This bacterium has a diverse repertoire of chemosensory systems, which represents a striking example of a survival mechanism in challenging environments. Two G. sulfurreducens MCP-GSU0582 and GSU0935-are members of a new family of chemotaxis sensor proteins containing a periplasmic PAS-like sensor domain with a c-type heme. Interestingly, the cellular location of this domain opens new routes to the understanding of the redox potential sensing signaling transduction pathways.
Keywords: Geobacter; c-type heme sensor domains; methyl-accepting chemotaxis proteins; redox-sensing; signal transduction; two-component system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

