Device Therapy in Chronic Heart Failure: JACC State-of-the-Art Review
- PMID: 34446165
- PMCID: PMC9941752
- DOI: 10.1016/j.jacc.2021.06.040
Device Therapy in Chronic Heart Failure: JACC State-of-the-Art Review
Abstract
The regulatory landscape for device-based heart failure (HF) therapies has seen a major shift in the last 7 years. In 2013, the U.S. Food and Drug Administration released guidance for early feasibility and first-in-human studies, thereby encouraging device innovation, and in 2016 the U.S. Congress authorized the Breakthrough Devices Program to expedite access for Americans to innovative devices indicated for diagnosis and treatment of serious illnesses, such as HF. Since December 2016, there has been an increase in the number of HF devices for which manufacturers are seeking approval through the breakthrough designation pathway. This has led to a rapid uptake in the development and evaluation of device-based HF therapies. This article reviews the current and future landscape of device therapies for chronic HF and associated comorbidities and the regulatory environment that is driving current and future innovation.
Keywords: U.S. Food and Drug Administration; device therapy; heart failure.
Copyright © 2021 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr Fudim has received support from National Heart, Lung, and Blood Institute (NHLBI) grant K23HL151744, American Heart Association grant 20IPA35310955, the Mario Family Award, the Duke Chair’s Award, the Translating Duke Health Award, Bayer, and BTG Specialty Pharmaceuticals; and has received consulting fees from AstraZeneca, AxonTherapies, CVRx, Daxor, Edwards LifeSciences, Galvani, and NXT Biomedical. Dr Abraham has received personal fees from Abbott during the conduct of the study; has received consulting fees from Boehringer Ingelheim, CVRx, Edwards Lifesciences, Impulse Dynamics, and Respicardia; has received salary support from V-Wave Medical; and has received research support from the NHLBI, all for studies performed within the heart failure arena. Dr von Bardeleben has received grants from Abbott Structural Heart. Dr Lindenfeld has received grant funding from AstraZeneca, Volumetrix, and Sensible Medical; and has received consulting fees from AstraZeneca, Abbott, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards LifeSciences, Impulse Dynamics, and V-Wave. Dr Ponikowski has received consulting fees and speaker honoraria from AstraZeneca, Boehringer Ingelheim, Vifor Pharma, Amgen, Servier, Novartis, Berlin Chemie, Bayer, Pfizer, Cibiem, Coridea, Impulse Dynamics, Renal Guard Solutions, BMS, and Abbott Vascular; is a co-principal investigator for the RESHAPE-HF trial for AbbottVascular; and has received a research grant from Vifor Pharma. Dr Sievert has served as a consultant for 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Celonova, Cibiem, CGuard, Comed BV, Contego, CVRx, Edwards, Endologix, Hemoteq, InspireMD, Lifetech, Maquet Getinge Group, Medtronic, Mitralign, Nuomao Medtech, Occlutech, pfm Medical, Recor, Renal Guard, Rox Medical, Terumo, Vascular Dynamics, Vivasure Medical, Venus, and Veryan. Dr Stone has received speaker or other honoraria from Cook, Terumo, QOOL Therapeutics, and Orchestra Biomed; has served as a consultant for Valfix, TherOx, Cardiomech, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme; and holds equity in or has options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. Dr Anker has received grants from Vifor Int and Abbott; and has received personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Abbott, Cardiac Dimensions, Impulse Dynamics, V-Wave, and Occlutech. Dr Butler has served as a consultant for Abbott, Adrenomed, American Regent, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, Cardiac Dimension, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Occlutech, Roche, and Vifor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
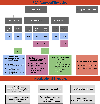
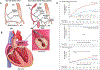


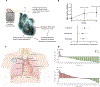
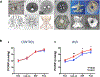
References
-
- US Department of Health and Human Services. Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies: Guidance for Industry and Food and Drug Administration Staff. 2013.
-
- Centers for Medicare & Medicaid Services. FY 2020 IPPS/LTCH PPS final rule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpat... (2019).
-
- Zile MR, Abraham WT, Lindenfeld J et al. First granted example of novel FDA trial design under Expedited Access Pathway for premarket approval: BeAT-HF. Am Heart J 2018;204:139–150. - PubMed
-
- Johnston JL, Dhruva SS, Ross JS, Rathi VK. Early experience with the FDA’s Breakthrough Devices program. Nat Biotechnol 2020;38:933–938. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

