Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma
- PMID: 34446469
- PMCID: PMC8576805
- DOI: 10.1183/13993003.01724-2021
Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma
Abstract
Background: Several randomised clinical trials have studied convalescent plasma for coronavirus disease 2019 (COVID-19) using different protocols, with different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralising antibody titres, at different time-points and severities of illness.
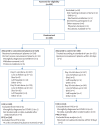
Methods: In the prospective multicentre DAWn-plasma trial, adult patients hospitalised with COVID-19 were randomised to 4 units of open-label convalescent plasma combined with standard of care (intervention group) or standard of care alone (control group). Plasma from donors with neutralising antibody titres (50% neutralisation titre (NT50)) ≥1/320 was the product of choice for the study.
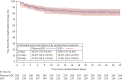
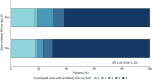
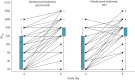
Results: Between 2 May 2020 and 26 January 2021, 320 patients were randomised to convalescent plasma and 163 patients to the control group according to a 2:1 allocation scheme. A median (interquartile range) volume of 884 (806-906) mL) convalescent plasma was administered and 80.68% of the units came from donors with neutralising antibody titres (NT50) ≥1/320. Median time from onset of symptoms to randomisation was 7 days. The proportion of patients alive and free of mechanical ventilation on day 15 was not different between both groups (convalescent plasma 83.74% (n=267) versus control 84.05% (n=137)) (OR 0.99, 95% CI 0.59-1.66; p=0.9772). The intervention did not change the natural course of antibody titres. The number of serious or severe adverse events was similar in both study arms and transfusion-related side-effects were reported in 19 out of 320 patients in the intervention group (5.94%).
Conclusions: Transfusion of 4 units of convalescent plasma with high neutralising antibody titres early in hospitalised COVID-19 patients did not result in a significant improvement of clinical status or reduced mortality.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: All authors report support for the present manuscript from the Belgian Healthcare Knowledge Center (KCE). Q. Van Thillo reports grants from Fonds Wetenschappelijk Onderzoek (FWO)–Vlaanderen Basic Research 2019–2021 outside the submitted work. G. Meyfroidt reports a FWO–Vlaanderen Senior Clinical Researcher Grant outside the submitted work.
Figures


Comment in
-
Convalescent plasma for SARS-CoV-2 infection: win or learn.Eur Respir J. 2022 Feb 10;59(2):2102076. doi: 10.1183/13993003.02076-2021. Print 2022 Feb. Eur Respir J. 2022. PMID: 34531275 Free PMC article.
References
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard with vaccination data. 2021. https://covid19.who.int Date last accessed: 16 July 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
