Spironolactone for adult female acne (SAFA): protocol for a double-blind, placebo-controlled, phase III randomised study of spironolactone as systemic therapy for acne in adult women
- PMID: 34446504
- PMCID: PMC8395279
- DOI: 10.1136/bmjopen-2021-053876
Spironolactone for adult female acne (SAFA): protocol for a double-blind, placebo-controlled, phase III randomised study of spironolactone as systemic therapy for acne in adult women
Abstract
Introduction: Acne is one of the most common inflammatory skin diseases worldwide and can have significant psychosocial impact and cause permanent scarring. Spironolactone, a potassium-sparing diuretic, has antiandrogenic properties, potentially reducing sebum production and hyperkeratinisation in acne-prone follicles. Dermatologists have prescribed spironolactone for acne in women for over 30 years, but robust clinical study data are lacking. This study seeks to evaluate whether spironolactone is clinically effective and cost-effective in treating acne in women.
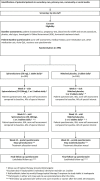
Methods and analysis: Women (≥18 years) with persistent facial acne requiring systemic therapy are randomised to receive one tablet per day of 50 mg spironolactone or a matched placebo until week 6, increasing to up to two tablets per day (total of 100 mg spironolactone or matched placebo) until week 24, along with usual topical therapy if desired. Study treatment stops at week 24; participants are informed of their treatment allocation and enter an unblinded observational follow-up period for up to 6 months (up to week 52 after baseline). Primary outcome is the Acne-specific Quality of Life (Acne-QoL) symptom subscale score at week 12. Secondary outcomes include Acne-QoL total and subscales; participant acne self-assessment recorded on a 6-point Likert scale at 6, 12, 24 weeks and up to 52 weeks; Investigator's Global Assessment at weeks 6 and 12; cost and cost effectiveness are assessed over 24 weeks. Aiming to detect a group difference of 2 points on the Acne-QoL symptom subscale (SD 5.8, effect size 0.35), allowing for 20% loss to follow-up, gives a sample size of 398 participants.
Ethics and dissemination: This protocol was approved by Wales Research Ethics Committee (18/WA/0420). Follow-up to be completed in early 2022. Findings will be disseminated to participants, peer-reviewed journals, networks and patient groups, on social media, on the study website and the Southampton Clinical Trials Unit website to maximise impact.
Trial registration number: ISRCTN12892056;Pre-results.
Keywords: acne; adult dermatology; dermatology.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Clinical and cost-effectiveness of spironolactone in treating persistent facial acne in women: SAFA double-blinded RCT.Health Technol Assess. 2024 Sep;28(56):1-86. doi: 10.3310/MYJT6804. Health Technol Assess. 2024. PMID: 39268864 Free PMC article. Clinical Trial.
-
Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial.BMJ. 2023 May 16;381:e074349. doi: 10.1136/bmj-2022-074349. BMJ. 2023. PMID: 37192767 Free PMC article. Clinical Trial.
-
Cost-effectiveness of Spironolactone for Adult Female Acne (SAFA): economic evaluation alongside a randomised controlled trial.BMJ Open. 2023 Dec 11;13(12):e073245. doi: 10.1136/bmjopen-2023-073245. BMJ Open. 2023. PMID: 38081673 Free PMC article. Clinical Trial.
-
Spironolactone for the Treatment of Moderate to Severe Acne in Adult Women: A Systematic Review and Meta-Analysis of Randomised Controlled Trials.Australas J Dermatol. 2025 May;66(3):165-168. doi: 10.1111/ajd.14428. Epub 2025 Feb 6. Australas J Dermatol. 2025. PMID: 39912292
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by
-
Scientometric analysis of trends in global research on acne treatment.Int J Womens Dermatol. 2023 Jul 28;9(3):e082. doi: 10.1097/JW9.0000000000000082. eCollection 2023 Oct. Int J Womens Dermatol. 2023. PMID: 37521754 Free PMC article.
-
Factors that influence women's enrolment and ongoing participation in a partially decentralised randomised controlled dermatology trial: a qualitative interview study with participants in the SAFA (Spironolactone for Adult Female Acne) trial.Trials. 2023 Oct 12;24(1):661. doi: 10.1186/s13063-023-07630-4. Trials. 2023. PMID: 37821899 Free PMC article. Clinical Trial.
-
Social media recruitment enhances participant diversity in dermatology clinical trial: findings from the SAFA trial.Trials. 2025 Aug 27;26(1):318. doi: 10.1186/s13063-025-08994-5. Trials. 2025. PMID: 40866885 Free PMC article.
-
Clinical and cost-effectiveness of spironolactone in treating persistent facial acne in women: SAFA double-blinded RCT.Health Technol Assess. 2024 Sep;28(56):1-86. doi: 10.3310/MYJT6804. Health Technol Assess. 2024. PMID: 39268864 Free PMC article. Clinical Trial.
-
Patient and Public Involvement in Research: Lessons for Inflammatory Bowel Disease.J Crohns Colitis. 2023 Nov 24;17(11):1882-1891. doi: 10.1093/ecco-jcc/jjad090. J Crohns Colitis. 2023. PMID: 37220886 Free PMC article. Review.
References
-
- NICE . Clinical knowledge summaries: acne vulgaris 2014.
-
- Tan J, Kang S, Leyden J. Prevalence and risk factors of acne scarring among patients consulting dermatologists in the USA. J Drugs Dermatol 2017;16:97–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical