Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement
- PMID: 34446702
- PMCID: PMC8390758
- DOI: 10.1038/s41467-021-25391-z
Implantable optical fibers for immunotherapeutics delivery and tumor impedance measurement
Abstract
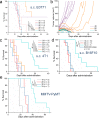
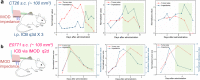
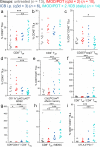
Immune checkpoint blockade antibodies have promising clinical applications but suffer from disadvantages such as severe toxicities and moderate patient-response rates. None of the current delivery strategies, including local administration aiming to avoid systemic toxicities, can sustainably supply drugs over the course of weeks; adjustment of drug dose, either to lower systemic toxicities or to augment therapeutic response, is not possible. Herein, we develop an implantable miniaturized device using electrode-embedded optical fibers with both local delivery and measurement capabilities over the course of a few weeks. The combination of local immune checkpoint blockade antibodies delivery via this device with photodynamic therapy elicits a sustained anti-tumor immunity in multiple tumor models. Our device uses tumor impedance measurement for timely presentation of treatment outcomes, and allows modifications to the delivered drugs and their concentrations, rendering this device potentially useful for on-demand delivery of potent immunotherapeutics without exacerbating toxicities.
© 2021. The Author(s).
Conflict of interest statement
A provisional patent (U.S. Patent Application No. 62/750,870) has been filed pertaining to the results presented in this paper by the authors (A.L.C., S.J., X.J., and R.T.). The authors declare no other competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

