Oral Fc-Coupled Preproinsulin Achieves Systemic and Thymic Delivery Through the Neonatal Fc Receptor and Partially Delays Autoimmune Diabetes
- PMID: 34447366
- PMCID: PMC8382691
- DOI: 10.3389/fimmu.2021.616215
Oral Fc-Coupled Preproinsulin Achieves Systemic and Thymic Delivery Through the Neonatal Fc Receptor and Partially Delays Autoimmune Diabetes
Abstract
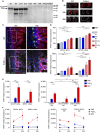
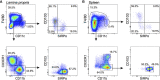
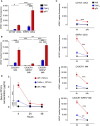
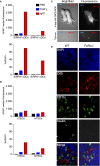
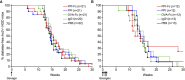
Tolerogenic vaccinations using beta-cell antigens are attractive for type 1 diabetes prevention, but clinical trials have been disappointing. This is probably due to the late timing of intervention, when multiple auto-antibodies are already present. We therefore devised a strategy to introduce the initiating antigen preproinsulin (PPI) during neonatal life, when autoimmunity is still silent and central tolerance mechanisms, which remain therapeutically unexploited, are more active. This strategy employs an oral administration of PPI-Fc, i.e. PPI fused with an IgG Fc to bind the intestinal neonatal Fc receptor (FcRn) that physiologically delivers maternal antibodies to the offspring during breastfeeding. Neonatal oral PPI-Fc vaccination did not prevent diabetes development in PPI T-cell receptor-transgenic G9C8.NOD mice. However, PPI-Fc was efficiently transferred through the intestinal epithelium in an Fc- and FcRn-dependent manner, was taken up by antigen presenting cells, and reached the spleen and thymus. Although not statistically significant, neonatal oral PPI-Fc vaccination delayed diabetes onset in polyclonal Ins2-/-.NOD mice that spontaneously develop accelerated diabetes. Thus, this strategy shows promise in terms of systemic and thymic antigen delivery via the intestinal FcRn pathway, but the current PPI-Fc formulation/regimen requires further improvements to achieve diabetes prevention.
Keywords: T cells; autoimmunity; immune tolerance; neonatal Fc receptor (FcRn); preproinsulin; thymus; type 1 diabetes; vaccination.
Copyright © 2021 Corcos, Culina, Deligne, Lavaud, You and Mallone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

