Mycobacterium tuberculosis Immune Response in Patients With Immune-Mediated Inflammatory Disease
- PMID: 34447382
- PMCID: PMC8382688
- DOI: 10.3389/fimmu.2021.716857
Mycobacterium tuberculosis Immune Response in Patients With Immune-Mediated Inflammatory Disease
Abstract
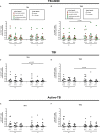
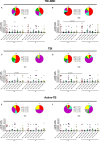
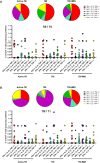
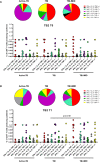
Subjects with immune-mediated inflammatory diseases (IMID), such as rheumatoid arthritis (RA), have an intrinsic higher probability to develop active-tuberculosis (TB) compared to the general population. The risk ranges from 2.0 to 8.9 in RA patients not receiving therapies. According to the WHO, the RA prevalence varies between 0.3% and 1% and is more common in women and in developed countries. Therefore, the identification and treatment of TB infection (TBI) in this fragile population is important to propose the TB preventive therapy. We aimed to study the M. tuberculosis (Mtb) specific T-cell response to find immune biomarkers of Mtb burden or Mtb clearance in patients with different TB status and different risk to develop active-TB disease. We enrolled TBI subjects as example of Mtb-containment, the active-TB as example of a replicating Mtb status, and the TBI-IMID as fragile population. To study the Mtb-specific response in a condition of possible Mtb sterilization, we longitudinally enrolled TBI subjects and active-TB patients before and after TB therapy. Peripheral blood mononuclear cells were stimulated overnight with Mtb peptides contained in TB1- and TB2-tubes of the Quantiferon-Plus kit. Then, we characterized by cytometry the Mtb-specific CD4 and CD8 T cells. In TBI-IMID, the TB therapy did not affect the ability of CD4 T cells to produce interferon-γ, tumor necrosis factor-α, and interleukin-2, their functional status, and their phenotype. The TB therapy determined a contraction of the triple functional CD4 T cells of the TBI subjects and active-TB patients. The CD45RA- CD27+ T cells stood out as a main subset of the Mtb-specific response in all groups. Before the TB-preventive therapy, the TBI subjects had higher proportion of Mtb-specific CD45RA-CD27+CD4+ T cells and the active-TB subjects had higher proportion of Mtb-specific CD45RA-CD27-CD4+ T cells compared to other groups. The TBI-IMID patients showed a phenotype similar to TBI, suggesting that the type of IMID and the IMID therapy did not affect the activation status of Mtb-specific CD4 T cells. Future studies on a larger and better-stratified TBI-IMID population will help to understand the change of the Mtb-specific immune response over time and to identify possible immune biomarkers of Mtb-containment or active replication.
Keywords: CD27; IFN-γ; M. tuberculosis; immune-mediated inflammatory disease; tuberculosis.
Copyright © 2021 Petruccioli, Petrone, Chiacchio, Farroni, Cuzzi, Navarra, Vanini, Massafra, Lo Pizzo, Guggino, Caccamo, Cantini, Palmieri and Goletti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- WHO . TB REPORT 2020. (2020). Available at: https://www.who.int/publications/i/item/9789240013131.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

