Analysis on the Characterization of Multiphoton Microscopy Images for Malignant Neoplastic Colon Lesion Detection under Deep Learning Methods
- PMID: 34447607
- PMCID: PMC8359734
- DOI: 10.4103/jpi.jpi_113_20
Analysis on the Characterization of Multiphoton Microscopy Images for Malignant Neoplastic Colon Lesion Detection under Deep Learning Methods
Abstract
Background: Colorectal cancer has a high incidence rate worldwide, with over 1.8 million new cases and 880,792 deaths in 2018. Fortunately, its early detection significantly increases the survival rate, reaching a cure rate of 90% when diagnosed at a localized stage. Colonoscopy is the gold standard technique for detection and removal of colorectal lesions with potential to evolve into cancer. When polyps are found in a patient, the current procedure is their complete removal. However, in this process, gastroenterologists cannot assure complete resection and clean margins which are given by the histopathology analysis of the removed tissue, which is performed at laboratory.
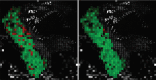
Aims: In this paper, we demonstrate the capabilities of multiphoton microscopy (MPM) technology to provide imaging biomarkers that can be extracted by deep learning techniques to identify malignant neoplastic colon lesions and distinguish them from healthy, hyperplastic, or benign neoplastic tissue, without the need for histopathological staining.
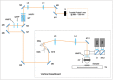
Materials and methods: To this end, we present a novel MPM public dataset containing 14,712 images obtained from 42 patients and grouped into 2 classes. A convolutional neural network is trained on this dataset and a spatially coherent predictions scheme is applied for performance improvement.
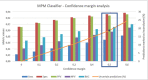
Results: We obtained a sensitivity of 0.8228 ± 0.1575 and a specificity of 0.9114 ± 0.0814 on detecting malignant neoplastic lesions. We also validated this approach to estimate the self-confidence of the network on its own predictions, obtaining a mean sensitivity of 0.8697 and a mean specificity of 0.9524 with the 18.67% of the images classified as uncertain.
Conclusions: This work lays the foundations for performing in vivo optical colon biopsies by combining this novel imaging technology together with deep learning algorithms, hence avoiding unnecessary polyp resection and allowing in situ diagnosis assessment.
Keywords: Colorectal polyps; convolutional neural network; dataset; multiphoton microscopy; optical biopsy.
Copyright: © 2021 Journal of Pathology Informatics.
Conflict of interest statement
There are no conflicts of interest.
Figures




References
-
- GLOBOCAN 2018 Database, Accessible Online at the Global Cancer Observatory. [Last accessed 2020 Oct 30]. Available from: https://gco.iarc.fr .
-
- SEER 18, Relative Survival by Stage (2010-2016) Database, Accessible Online At. [Last accessed 2020 Oct 30]. Available from: https://seer.cancer.gov/canques/survival.html .
-
- Rajasekhar PT, Mason J, Wilson A, Close H, Rutter M, Saunders B, et al. Detect inspect characterise resect and discard 2: Are we ready to dispense with histology? Gut. 2015;64:A13.
-
- Kaltenbach T, Rastogi A, Rouse RV, McQuaid KR, Sato T, Bansal A, et al. Real-time optical diagnosis for diminutive colorectal polyps using narrow-band imaging: The VALID randomised clinical trial. Gut. 2015;64:1569–77. - PubMed
-
- Hale MF, Kurien M, Basumani P, Slater R, Sanders DS, Hopper AD. Endoscopy II: PTU-233 In vivo polyp size and histology assessment at colonoscopy: Are we ready to resect and discard.A multi-centre analysis of 1212 polypectomies? Gut. 2012;61:A280–1.
LinkOut - more resources
Full Text Sources

