Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity
- PMID: 34448031
- PMCID: PMC8712294
- DOI: 10.1007/s00259-021-05474-1
Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity
Abstract
Purpose: The aim of this retrospective analysis is to estimate the most appropriate single cycle and cumulative doses of 225Ac-DOTATOC in patients treated for somatostatin-receptor-expressing cancers.
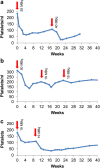
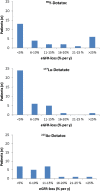
Methods: 225Ac-DOTATOC was administered to thirty-nine patients with various somatostatin-receptor-positive tumors. Baseline and follow-up 68Ga-DOTATOC PET/CT, lab tests, and renal scintigraphy were obtained. Patients received long-term follow-up either at the local cancer center or in close collaboration with external oncologists. Acute and chronic hematological toxicity was evaluated quantitatively over time. Long-term follow-up of creatinine was used to approximate the annual loss of estimated GFR (eGFR).
Results: Dose-dependent acute hematological toxicity was seen at single doses above 40 MBq or repeated doses greater than approximately 20 MBq 225Ac-DOTATOC at 4 month intervals. Treatment-related kidney failure occurred in 2 patients after a delay of >4 years but was independent of administered radioactivity, and other clinical risk factors were important contributors to renal decline. In general, the annual decline of eGFR among patients did not follow a clear dose-effect relationship even in patients with previous β-therapy. An average eGFR-loss of 8.4ml/min (9.9%) per year was observed which is similar to the experience with β-therapy studies.
Conclusion: Treatment activities of approx. 20 MBq per cycle (4 monthly repetition) and cumulative doses up to 60-80 MBq generally avoided both acute and chronic grade 3/4 hematotoxicity in patients with advanced stage malignancies. Chronic renal toxicity was observed at these doses, but pre-existing renal risk factors were important co-factors. These data represent a starting point for additional research to more precisely define safety thresholds of 225Ac-DOTATOC.
Keywords: Ac-225; Neuroendocrine tumor; PRRT; TAT; Targeted α therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
90Y-DOTATOC Dosimetry-Based Personalized Peptide Receptor Radionuclide Therapy.J Nucl Med. 2018 Nov;59(11):1692-1698. doi: 10.2967/jnumed.117.202903. Epub 2018 Mar 9. J Nucl Med. 2018. PMID: 29523629 Free PMC article. Clinical Trial.
-
Yttrium-90 DOTATOC: first clinical results.Eur J Nucl Med. 1999 Nov;26(11):1439-47. Eur J Nucl Med. 1999. PMID: 10552085
-
Prospective Within-Patient Assessment of the Impact of an Unlabeled Octreotide Pre-dose on the Biodistribution and Tumor Uptake of 68Ga DOTATOC as Assessed by Dynamic Whole-body PET in Patients with Neuroendocrine Tumors: Implications for Diagnosis and Therapy.Mol Imaging Biol. 2021 Oct;23(5):766-774. doi: 10.1007/s11307-021-01600-5. Epub 2021 Apr 7. Mol Imaging Biol. 2021. PMID: 33829361
-
Differentiated thyroid cancer: a new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients.Thyroid. 2014 Apr;24(4):715-26. doi: 10.1089/thy.2013.0225. Epub 2013 Nov 14. Thyroid. 2014. PMID: 24102584 Clinical Trial.
-
Yttrium-90 DOTATOC therapy in GEP-NET and other SST2 expressing tumors: a selected review.Ann Nucl Med. 2011 Feb;25(2):75-85. doi: 10.1007/s12149-010-0444-0. Epub 2010 Nov 25. Ann Nucl Med. 2011. PMID: 21107762 Review.
Cited by
-
Preclinical Evaluation of 225Ac-Labeled Single-Domain Antibody for the Treatment of HER2pos Cancer.Mol Cancer Ther. 2022 Dec 2;21(12):1835-1845. doi: 10.1158/1535-7163.MCT-21-1021. Mol Cancer Ther. 2022. PMID: 36129807 Free PMC article.
-
Multiplexed Imaging Reveals the Spatial Relationship of the Extracellular Acidity-Targeting pHLIP with Necrosis, Hypoxia, and the Integrin-Targeting cRGD Peptide.Cells. 2022 Nov 4;11(21):3499. doi: 10.3390/cells11213499. Cells. 2022. PMID: 36359894 Free PMC article.
-
Advances in Radioligand Theranostics in Oncology.Mol Diagn Ther. 2024 May;28(3):265-289. doi: 10.1007/s40291-024-00702-4. Epub 2024 Mar 31. Mol Diagn Ther. 2024. PMID: 38555542 Review.
-
Expanding Theranostic Radiopharmaceuticals for Tumor Diagnosis and Therapy.Pharmaceuticals (Basel). 2021 Dec 22;15(1):13. doi: 10.3390/ph15010013. Pharmaceuticals (Basel). 2021. PMID: 35056071 Free PMC article. Review.
-
Clinical application of targeted α-emitter therapy in gastroenteropancreatic neuroendocrine neoplasms.J Gastroenterol. 2025 Jul;60(7):809-819. doi: 10.1007/s00535-025-02241-z. Epub 2025 Apr 12. J Gastroenterol. 2025. PMID: 40220045 Free PMC article. Review.
References
-
- Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

