Melatonin modulates nitric oxide-regulated WNK-SPAK/OSR1-NKCC1 signaling in dorsal raphe nucleus of rats
- PMID: 34448462
- PMCID: PMC8405441
- DOI: 10.4196/kjpp.2021.25.5.449
Melatonin modulates nitric oxide-regulated WNK-SPAK/OSR1-NKCC1 signaling in dorsal raphe nucleus of rats
Abstract
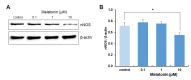
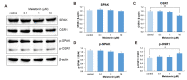
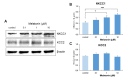
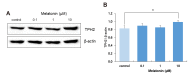
The sleep-wake cycle is regulated by the alternating activity of sleep- and wake-promoting neurons. The dorsal raphe nucleus (DRN) secretes 5-hydroxytryptamine (5-HT, serotonin), promoting wakefulness. Melatonin secreted from the pineal gland also promotes wakefulness in rats. Our laboratory recently demonstrated that daily changes in nitric oxide (NO) production regulates a signaling pathway involving with-no-lysine kinase (WNK), Ste20-related proline alanine rich kinase (SPAK)/oxidative stress response kinase 1 (OSR1), and cation-chloride co-transporters (CCC) in rat DRN serotonergic neurons. This study was designed to investigate the effect of melatonin on NO-regulated WNK-SPAK/OSR1-CCC signaling in wake-inducing DRN neurons to elucidate the mechanism underlying melatonin's wake-promoting actions in rats. Ex vivo treatment of DRN slices with melatonin suppressed neuronal nitric oxide synthase (nNOS) expression and increased WNK4 expression without altering WNK1, 2, or 3. Melatonin increased phosphorylation of OSR1 and the expression of sodium-potassium-chloride co-transporter 1 (NKCC1), while potassium-chloride cotransporter 2 (KCC2) remained unchanged. Melatonin increased the expression of tryptophan hydroxylase 2 (TPH2, serotonin-synthesizing enzyme). The present study suggests that melatonin may promote its wakefulness by modulating NO-regulated WNK-SPAK/OSR1-KNCC1 signaling in rat DRN serotonergic neurons.
Keywords: Dorsal raphe nucleus; Melatonin; Nitric oxide; Sodium potassium chloride cotransporter; WNK.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Role of nitric oxide and WNK-SPAK/OSR1-KCC2 signaling in daily changes in GABAergic inhibition in the rat dorsal raphe neurons.Neuropharmacology. 2018 Jun;135:355-367. doi: 10.1016/j.neuropharm.2018.03.035. Epub 2018 Mar 27. Neuropharmacology. 2018. PMID: 29596900
-
Suppression of WNK1-SPAK/OSR1 Attenuates Bone Cancer Pain by Regulating NKCC1 and KCC2.J Pain. 2019 Dec;20(12):1416-1428. doi: 10.1016/j.jpain.2019.05.005. Epub 2019 May 11. J Pain. 2019. PMID: 31085334
-
WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia.Am J Physiol Renal Physiol. 2020 Jan 1;318(1):F216-F228. doi: 10.1152/ajprenal.00232.2019. Epub 2019 Nov 18. Am J Physiol Renal Physiol. 2020. PMID: 31736353 Free PMC article.
-
Targeting the WNK-SPAK/OSR1 Pathway and Cation-Chloride Cotransporters for the Therapy of Stroke.Int J Mol Sci. 2021 Jan 27;22(3):1232. doi: 10.3390/ijms22031232. Int J Mol Sci. 2021. PMID: 33513812 Free PMC article. Review.
-
WNK-SPAK/OSR1 signaling: lessons learned from an insect renal epithelium.Am J Physiol Renal Physiol. 2018 Oct 1;315(4):F903-F907. doi: 10.1152/ajprenal.00176.2018. Epub 2018 Jun 20. Am J Physiol Renal Physiol. 2018. PMID: 29923766 Free PMC article. Review.
Cited by
-
The Interplay Between Melatonin and Nitric Oxide: Mechanisms and Implications in Stroke Pathophysiology.Antioxidants (Basel). 2025 Jun 13;14(6):724. doi: 10.3390/antiox14060724. Antioxidants (Basel). 2025. PMID: 40563356 Free PMC article. Review.
-
Sex-specific modulation of the medial prefrontal cortex by glutamatergic median raphe neurons.Sci Adv. 2023 Nov 10;9(45):eadg4800. doi: 10.1126/sciadv.adg4800. Epub 2023 Nov 10. Sci Adv. 2023. PMID: 37948526 Free PMC article.
-
Median raphe glutamatergic neuron-mediated enhancement of GABAergic transmission and suppression of long-term potentiation in the hippocampus.Heliyon. 2024 Sep 19;10(19):e38192. doi: 10.1016/j.heliyon.2024.e38192. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39386853 Free PMC article.

