Angiotensin II biphasically regulates cell differentiation in human iPSC-derived kidney organoids
- PMID: 34448643
- PMCID: PMC8616599
- DOI: 10.1152/ajprenal.00134.2021
Angiotensin II biphasically regulates cell differentiation in human iPSC-derived kidney organoids
Abstract
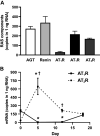
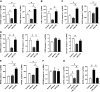
Human kidney organoid technology holds promise for novel kidney disease treatment strategies and utility in pharmacological and basic science. Given the crucial roles of the intrarenal renin-angiotensin system (RAS) and angiotensin II (ANG II) in the progression of kidney development and injury, we investigated the expression of RAS components and effects of ANG II on cell differentiation in human kidney organoids. Human induced pluripotent stem cell-derived kidney organoids were induced using a modified 18-day Takasato protocol. Gene expression analysis by digital PCR and immunostaining demonstrated the formation of renal compartments and expression of RAS components. The ANG II type 1 receptor (AT1R) was strongly expressed in the early phase of organoid development (around day 0), whereas ANG II type 2 receptor (AT2R) expression levels peaked on day 5. Thus, the organoids were treated with 100 nM ANG II in the early phase on days 0-5 (ANG II-E) or during the middle phase on days 5-10 (ANG II-M). ANG II-E was observed to decrease levels of marker genes for renal tubules and proximal tubules, and the downregulation of renal tubules was inhibited by an AT1R antagonist. In contrast, ANG II-M increased levels of markers for podocytes, the ureteric tip, and the nephrogenic mesenchyme, and an AT2R blocker attenuated the ANG II-M-induced augmentation of podocyte formation. These findings demonstrate RAS expression and ANG II exertion of biphasic effects on cell differentiation through distinct mediatory roles of AT1R and AT2R, providing a novel strategy to establish and further characterize the developmental potential of human induced pluripotent stem cell-derived kidney organoids.NEW & NOTEWORTHY This study demonstrates angiotensin II exertion of biphasic effects on cell differentiation through distinct mediatory roles of angiotensin II type 1 receptor and type 2 receptor in human induced pluripotent stem cell-derived kidney organoids, providing a novel strategy to establish and further characterize the developmental potential of the human kidney organoids.
Keywords: angiotensin II; angiotensinogen; induced pluripotent stem cells; kidney organoids.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

