Computational models in the service of X-ray and cryo-electron microscopy structure determination
- PMID: 34449113
- PMCID: PMC8616789
- DOI: 10.1002/prot.26223
Computational models in the service of X-ray and cryo-electron microscopy structure determination
Abstract
Critical assessment of structure prediction (CASP) conducts community experiments to determine the state of the art in computing protein structure from amino acid sequence. The process relies on the experimental community providing information about not yet public or about to be solved structures, for use as targets. For some targets, the experimental structure is not solved in time for use in CASP. Calculated structure accuracy improved dramatically in this round, implying that models should now be much more useful for resolving many sorts of experimental difficulties. To test this, selected models for seven unsolved targets were provided to the experimental groups. These models were from the AlphaFold2 group, who overall submitted the most accurate predictions in CASP14. Four targets were solved with the aid of the models, and, additionally, the structure of an already solved target was improved. An a posteriori analysis showed that, in some cases, models from other groups would also be effective. This paper provides accounts of the successful application of models to structure determination, including molecular replacement for X-ray crystallography, backbone tracing and sequence positioning in a cryo-electron microscopy structure, and correction of local features. The results suggest that, in future, there will be greatly increased synergy between computational and experimental approaches to structure determination.
Keywords: CASP; X-ray crystallography; cryo-EM; protein structure prediction.
© 2021 Wiley Periodicals LLC.
Figures

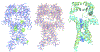


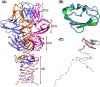



References
-
- Tramontano A The role of molecular modelling in biomedical research. FEBS Lett. 2006;580(12):2928–2934. - PubMed

