Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation
- PMID: 34449593
- PMCID: PMC8395836
- DOI: 10.3390/gels7030122
Alumina-Doped Silica Aerogels for High-Temperature Thermal Insulation
Abstract
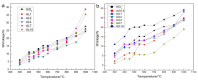
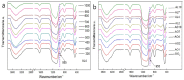
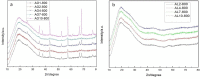
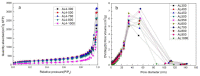
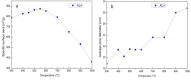
In this study, we used two methods to prepare alumina-doped silica aerogels with the aim of increasing the thermal stability of silica aerogels. The first method was physical doping of α-Al2O3 nano powders, and the second method was to create a chemical compound via the co-precursor of TEOS and AlCl3·6H2O in different proportions. The shrinkage, chemical composition, and specific surface area (SSA) of samples after heating at different temperatures were analyzed. Our results show that the silicon hydroxyl groups of samples derived from AlCl3·6H2O gradually decreased and nearly disappeared after heating at 800 °C, which indicates the complete dehydration of the silicon hydroxyl. Thus, the samples exhibited a large linear shrinkage and decreased SSA after high-temperature heat treatment. By contrast, samples doped with α-Al2O3 powders retained abundant silicon hydroxyl groups, and the 6.1 wt.% α-Al2O3-doped sample exhibited the lowest linear shrinkage of 11% and the highest SSA of 1056 m2/g after heat treatment at 800 °C. The alumina-doped silica aerogels prepared using a simple and low-price synthesized method pave the way for the low-cost and large-scale production of high-temperature thermal insulation.
Keywords: alumina-doped; high temperature resistance; silica aerogels; thermal stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Despetis F., Calas-Etienne S., Etienne P. Slow crack growth in silica aerogels: A review. J. Sol.-Gel Sci. Technol. 2019;90:20–27. doi: 10.1007/s10971-018-4857-x. - DOI
-
- Parale V.G., Lee K.Y., Park H.H. Flexible and transparent silica aerogels: An overview. J. Korean Ceram. Soc. 2017;54:184–199. doi: 10.4191/kcers.2017.54.3.12. - DOI
-
- He Y., Xie T. A review of heat transfer models of nanoporous silica aerogel insulation material. Chin. Sci. Bull. 2015;60:137–163.
-
- Pisal A.A., Rao A.V. Comparative studies on the physical properties of TEOS, TMOS and Na2SiO3 based silica aerogels by ambient pressure drying method. J. Porous Mater. 2016;23:1547–1556. doi: 10.1007/s10934-016-0215-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

