The Utility of Pharmacogenetic-Guided Psychotropic Medication Selection for Pediatric Patients: A Retrospective Study
- PMID: 34449718
- PMCID: PMC8396342
- DOI: 10.3390/pediatric13030049
The Utility of Pharmacogenetic-Guided Psychotropic Medication Selection for Pediatric Patients: A Retrospective Study
Abstract
Background: To describe trends and clinical experiences in applying commercial pharmacogenetic testing among pediatric patients with neuropsychiatric disorders.
Methods: Demographic and clinical data of patients receiving GeneSight® testing from January 2015 to November 2016 at an urban pediatric hospital were retrospectively extracted from medical charts. Outcome data included pharmacogenetic test results and medication prescriptions before and after the test.
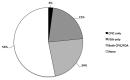
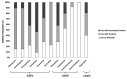
Results: A total of 450 patients (12.1 ± 4.3 years) diagnosed with anxiety disorder, attention deficit hyperactivity disorder, developmental disorders including autism, and/or a mood disorder received testing, and 435 of them were prescribed medications. Comparing data before and after testing, the total number of psychotropic prescriptions were reduced by 27.2% and the number of prescribed medications with severe gene-drug interactions decreased from 165 to 95 (11.4% to 8.9% of total medications prescribed). Approximately 40% of actionable genetic annotation were related to CYP2CD6 and CYP2C19. Patients of Asian descent had significantly higher likelihood than other races of being classified as poor to intermediate metabolizers of antidepressants, mood stabilizers, and antipsychotics (p = 0.008, 0.007, and 0.001, respectively). Diagnoses, including autism spectrum disorder, were not associated with increased risks of severe gene-drug interactions.
Conclusions: Pharmacogenetic testing in child and adolescent psychiatry is currently based on few clinically actionable genes validated by CPIC and/or FDA. Although this approach can be moderately utilized to guide psychotropic medication prescribing for pediatric patients with psychiatric disorders, clinicians should cautiously interpret test results while still relying on clinical experience and judgment to direct the final selection of medication.
Keywords: CPIC/FDA; pharmacogenetic testing; practice recommendations; psychiatric disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Danielson M.L., Bitsko R.H., Ghandour R.M., Holbrook J.R., Kogan M.D., Blumberg S.J. Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment Among U.S. Children and Adolescents, 2016. J. Clin. Child Adolesc. Psychol. 2018;47:199–212. doi: 10.1080/15374416.2017.1417860. - DOI - PMC - PubMed
-
- CDC Children’s Mental Health. [(accessed on 21 January 2020)]; Available online: https://www.cdc.gov/childrensmentalhealth/data.html.
LinkOut - more resources
Full Text Sources

