CSF Proteomic Alzheimer's Disease-Predictive Subtypes in Cognitively Intact Amyloid Negative Individuals
- PMID: 34449748
- PMCID: PMC8396164
- DOI: 10.3390/proteomes9030036
CSF Proteomic Alzheimer's Disease-Predictive Subtypes in Cognitively Intact Amyloid Negative Individuals
Abstract
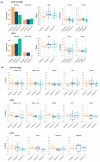
We recently discovered three distinct pathophysiological subtypes in Alzheimer's disease (AD) using cerebrospinal fluid (CSF) proteomics: one with neuronal hyperplasticity, a second with innate immune system activation, and a third subtype with blood-brain barrier dysfunction. It remains unclear whether AD proteomic subtype profiles are a consequence of amyloid aggregation, or might exist upstream from aggregated amyloid. We studied this question in 127 older individuals with intact cognition and normal AD biomarkers in two independent cohorts (EMIF-AD MBD and ADNI). We clustered 705 proteins measured in CSF that were previously related to AD. We identified in these cognitively intact individuals without AD pathology three subtypes: two subtypes were seen in both cohorts (n = 49 with neuronal hyperplasticity and n = 44 with blood-brain barrier dysfunction), and one only in ADNI (n = 12 with innate immune activation). The proteins specific for these subtypes strongly overlapped with AD subtype protein profiles (overlap coefficients 92%-71%). Longitudinal p181-tau and amyloid β 1-42 (Aβ42) CSF analysis showed that in the hyperplasticity subtype p181-tau increased (β = 2.6 pg/mL per year, p = 0.01) and Aβ42 decreased over time (β = -4.4 pg/mL per year, p = 0.03), in the innate immune activation subtype p181-tau increased (β = 3.1 pg/mL per year, p = 0.01) while in the blood-brain barrier dysfunction subtype Aβ42 decreased (β = -3.7 pg/mL per year, p = 0.009). These findings suggest that AD proteomic subtypes might already manifest in cognitively normal individuals and may predispose for AD before amyloid has reached abnormal levels.
Keywords: Alzheimer’s disease; amyloid beta; cerebrospinal fluid proteomics; cognitive functioning; risk factors; tau.
Conflict of interest statement
H.Z. has served at scientific advisory boards for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, and CogRx, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. The other authors declare no conflict of interest.
Figures

References
-
- Albert M.S., DeKosky S., Dickson D.W., Dubois B., Feldman H., Fox N., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. - DOI - PMC - PubMed
-
- Dubois B., Feldman H.H., Jacova C., DeKosky S.T., Barberger-Gateau P., Cummings J.L., Delacourte A., Galasko D., Gauthier S., Jicha G.A., et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. - DOI - PubMed
-
- Jack C.R., Jr., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C., Thies W., Phelps C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. - DOI - PMC - PubMed
-
- Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

