Effects of captopril against radiation injuries in the Göttingen minipig model of hematopoietic-acute radiation syndrome
- PMID: 34449797
- PMCID: PMC8396780
- DOI: 10.1371/journal.pone.0256208
Effects of captopril against radiation injuries in the Göttingen minipig model of hematopoietic-acute radiation syndrome
Abstract
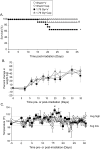
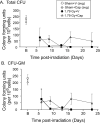
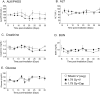
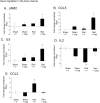
Our laboratory has demonstrated that captopril, an angiotensin converting enzyme inhibitor, mitigates hematopoietic injury following total body irradiation in mice. Improved survival in mice is correlated with improved recovery of mature blood cells and bone marrow, reduction of radiation-induced inflammation, and suppression of radiation coagulopathy. Here we investigated the effects of captopril treatment against radiation injuries in the Göttingen mini pig model of Hematopoietic-Acute Radiation Syndrome (H-ARS). Minipigs were given captopril orally (0.96 mg/kg) twice daily for 12 days following total body irradiation (60Co 1.79 Gy, 0.42-0.48 Gy/min). Blood was drawn over a time course following irradiation, and tissue samples were collected at euthanasia (32-35 days post-irradiation). We observed improved survival with captopril treatment, with survival rates of 62.5% in vehicle treated and 87.5% in captopril treated group. Additionally, captopril significantly improved recovery of peripheral blood mononuclear cells, and a trend toward improvement in recovery of red blood cells and platelets. Captopril significantly reduced radiation-induced expression of cytokines erythropoietin and granulocyte-macrophage colony-stimulating factor and suppressed radiation-induced acute-phase inflammatory response cytokine serum amyloid protein A. Using quantitative-RT-PCR to monitor bone marrow recovery, we observed significant suppression of radiation-induced expression of redox stress genes and improved hematopoietic cytokine expression. Our findings suggest that captopril activities in the Göttingen minipig model of hematopoietic-acute radiation syndrome reflect findings in the murine model.
Conflict of interest statement
KOG is currently employed by Leidos Biomedical Research Inc. KOG was not employed by Leidos Biomedical Research Inc at the time this study was conducted. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- World Nuclear Association (2019) World Nuclear Energy Performance 2019, https://www.world-nuclear.org/getmedia/d77ef8a1-b720-44aa-9b87-abf09f474....
-
- Guidance Document. Product Development Under the Animal Rule. Rockville, MD. Food And Drug Administration. 2015 November. Docket Number FDA-2009-D-0007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

