Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic
- PMID: 34450028
- PMCID: PMC8489356
- DOI: 10.1016/j.cell.2021.08.002
Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic
Erratum in
-
Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic.Cell. 2024 Mar 14;187(6):1565-1566. doi: 10.1016/j.cell.2024.02.015. Cell. 2024. PMID: 38490183 Free PMC article. No abstract available.
Abstract
GRN mutations cause frontotemporal dementia (GRN-FTD) due to deficiency in progranulin (PGRN), a lysosomal and secreted protein with unclear function. Here, we found that Grn-/- mice exhibit a global deficiency in bis(monoacylglycero)phosphate (BMP), an endolysosomal phospholipid we identified as a pH-dependent PGRN interactor as well as a redox-sensitive enhancer of lysosomal proteolysis and lipolysis. Grn-/- brains also showed an age-dependent, secondary storage of glucocerebrosidase substrate glucosylsphingosine. We investigated a protein replacement strategy by engineering protein transport vehicle (PTV):PGRN-a recombinant protein linking PGRN to a modified Fc domain that binds human transferrin receptor for enhanced CNS biodistribution. PTV:PGRN rescued various Grn-/- phenotypes in primary murine macrophages and human iPSC-derived microglia, including oxidative stress, lysosomal dysfunction, and endomembrane damage. Peripherally delivered PTV:PGRN corrected levels of BMP, glucosylsphingosine, and disease pathology in Grn-/- CNS, including microgliosis, lipofuscinosis, and neuronal damage. PTV:PGRN thus represents a potential biotherapeutic for GRN-FTD.
Keywords: GBA; LBPA; galectin-3; lipidomics; lipids; lipofuscin; lysobisphosphatidic acid; lysosome; metabolomics; neurodegenerative disease.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors, except A.L.B., H.H., H.J.R., and B.F.B., are full-time employees and/or shareholders of Denali Therapeutics. A.L.B. reports consultancy for AGTC, Alector, Arkuda, Arvinas, Asceneuron, AZTherapies, Bioage, GSK, Humana, Lundbeck, Ono, Roche, Samumed, Sangamo, Stealth Therapeutics, Third Rock, Transposon, UCB, and Wave and research support from the Association for Frontotemporal Degeneration, Biogen, Bluefield Project to Cure Frontotemporal Dementia, Eli Lilly, Eisai, National Institutes of Health (grant numbers U19AG063911, U54NS092089, R01AG031278), and the Rainwater Charitable Foundation. B.F.B. reports research support from the NIH (U19AG063911). H.J.R. reports funding from the NIH and consultancy with Alector, Ionis, Biogen, Wave, Takeda, and AFTD. This work has been in part described in one or more pending patent applications.
Figures

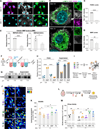
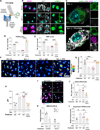
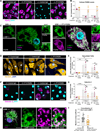
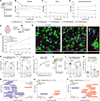


References
-
- Andreone BJ, Przybyla L, Llapashtica C, Rana A, Davis SS, van Lengerich B, Lin K, Shi J, Mei Y, Astarita G, et al. (2020). Alzheimer’s-associated PLCgamma2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat Neurosci 23, 927–938. - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. (2006). Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

