Painful diabetic neuropathy leads to functional CaV3.2 expression and spontaneous activity in skin nociceptors of mice
- PMID: 34450183
- PMCID: PMC8549116
- DOI: 10.1016/j.expneurol.2021.113838
Painful diabetic neuropathy leads to functional CaV3.2 expression and spontaneous activity in skin nociceptors of mice
Abstract
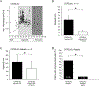
Painful diabetic neuropathy occurs in approximately 20% of diabetic patients with underlying pathomechanisms not fully understood. We evaluated the contribution of the CaV3.2 isoform of T-type calcium channel to hyperglycemia-induced changes in cutaneous sensory C-fiber functions and neuropeptide release employing the streptozotocin (STZ) diabetes model in congenic mouse strains including global knockouts (KOs). Hyperglycemia established for 3-5 weeks in male C57BL/6J mice led to major reorganizations in peripheral C-fiber functions. Unbiased electrophysiological screening of mechanosensitive single-fibers in isolated hairy hindpaw skin revealed a relative loss of (polymodal) heat sensing in favor of cold sensing. In healthy CaV3.2 KO mice both heat and cold sensitivity among the C-fibers seemed underrepresented in favor of exclusive mechanosensitivity, low-threshold in particular, which deficit became significant in the diabetic KOs. Diabetes also led to a marked increase in the incidence of spontaneous discharge activity among the C-fibers of wildtype mice, which was reduced by the specific CaV3.2 blocker TTA-P2 and largely absent in the KOs. Evaluation restricted to the peptidergic class of nerve fibers - measuring KCl-stimulated CGRP release - revealed a marked reduction in the sciatic nerve by TTA-P2 in healthy but not diabetic wildtypes, the latter showing CGRP release that was as much reduced as in healthy and, to the same extent, in diabetic CaV3.2 KOs. These data suggest that diabetes abrogates all CaV3.2 functionality in the peripheral nerve axons. In striking contrast, diabetes markedly increased the KCl-stimulated CGRP release from isolated hairy skin of wildtypes but not KO mice, and TTA-P2 reversed this increase, strongly suggesting a de novo expression of CaV3.2 in peptidergic cutaneous nerve endings which may contribute to the enhanced spontaneous activity. De-glycosylation by neuraminidase showed clear desensitizing effects, both in regard to spontaneous activity and stimulated CGRP release, but included actions independent of CaV3.2. However, as diabetes-enhanced glycosylation is decisive for intra-axonal trafficking, it may account for the substantial reorganizations of the CaV3.2 distribution. The results may strengthen the validation of CaV3.2 channel as a therapeutic target of treating painful diabetic neuropathy.
Keywords: Calcitonin gene-related peptide; Excitability; Neuropathic pain; Neuropeptide release; Sciatic nerve; T-type calcium channel; TTA-P2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

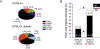


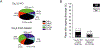

References
-
- Abad-Rodríguez J, Díez-Revuelta N, 2015. Axon glycoprotein routing in nerve polarity, function, and repair. Trends in biochemical sciences 40, 385–396. - PubMed
-
- Amrutkar DV, Ploug KB, Olesen J, Jansen-Olesen I, 2011. Role for voltage gated calcium channels in calcitonin gene-related peptide release in the rat trigeminovascular system. Neuroscience 172, 510–517. - PubMed
-
- Ayoola C, Hwang SM, Hong SJ, Rose KE, Boyd C, Bozic N, Park JY, Osuru HP, DiGruccio MR, Covey DF, Jevtovic-Todorovic V, Todorovic SM, 2014. Inhibition of CaV3.2 T-type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone. Psychopharmacology 231, 3503–3515. - PMC - PubMed
-
- Babes A, Fischer MJ, Reid G, Sauer SK, Zimmermann K, Reeh PW, 2010. Electrophysiological and neurochemical techniques to investigate sensory neurons in analgesia research. Methods Mol. Biol 617:237–59., 237–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

