Bi-directional gene activation and repression promote ASC differentiation and enhance bone healing in osteoporotic rats
- PMID: 34450254
- PMCID: PMC8753367
- DOI: 10.1016/j.ymthe.2021.08.024
Bi-directional gene activation and repression promote ASC differentiation and enhance bone healing in osteoporotic rats
Abstract
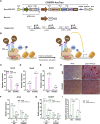
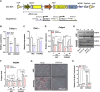
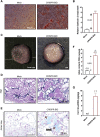
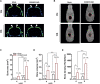
Calvarial bone healing is challenging, especially for individuals with osteoporosis because stem cells from osteoporotic patients are highly prone to adipogenic differentiation. Based on previous findings that chondrogenic induction of adipose-derived stem cells (ASCs) can augment calvarial bone healing, we hypothesized that activating chondroinductive Sox Trio genes (Sox5, Sox6, Sox9) and repressing adipoinductive genes (C/ebp-α, Ppar-γ) in osteoporotic ASCs can reprogram cell differentiation and improve calvarial bone healing after implantation. However, simultaneous gene activation and repression in ASCs is difficult. To tackle this problem, we built a CRISPR-BiD system for bi-directional gene regulation. Specifically, we built a CRISPR-AceTran system that exploited both histone acetylation and transcription activation for synergistic Sox Trio activation. We also developed a CRISPR interference (CRISPRi) system that exploited DNA methylation for repression of adipoinductive genes. We combined CRISPR-AceTran and CRISPRi to form the CRISPR-BiD system, which harnessed three mechanisms (transcription activation, histone acetylation, and DNA methylation). After delivery into osteoporotic rat ASCs, CRISPR-BiD significantly enhanced chondrogenesis and in vitro cartilage formation. Implantation of the engineered osteoporotic ASCs into critical-sized calvarial bone defects significantly improved bone healing in osteoporotic rats. These results implicated the potential of the CRISPR-BiD system for bi-directional regulation of cell fate and regenerative medicine.
Keywords: CRISPR-AceTran; CRISPR-BiD; CRISPRi; bi-direction gene regulation; regenerative medicine.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Szpalski C., Barr J., Wetterau M., Saadeh P.B., Warren S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus. 2010;29:E8. - PubMed
-
- Ajai S., Sabir A., Abbas M., Srivastava R.N. Evaluation of role of microRNA in osteoporosis. J. Pharm. Biomed. Sci. 2013;33:1439–1448.
-
- Lin C.-Y., Lin K.-J., Kao C.-Y., Chen M.-C., Lo W.H., Yen T.C., Chang Y.H., Hu Y.C. The role of adipose-derived stem cells engineered with the persistently expressing hybrid baculovirus in the healing of massive bone defects. Biomaterials. 2011;32:6505–6514. - PubMed
-
- Lu C.-H., Lin K.-J., Chiu H.-Y., Chen C.-Y., Yen T.-C., Hwang S.-M., Chang Y.H., Hu Y.C. Improved chondrogenesis and engineered cartilage formation from TGF-β3-expressing adipose-derived stem cells cultured in the rotating-shaft bioreactor. Tissue Eng. Part A. 2012;18:2114–2124. - PubMed
-
- Lin C.-Y., Chang Y.-H., Kao C.-Y., Lu C.-H., Sung L.-Y., Yen T.-C., Lin K.J., Hu Y.C. Augmented healing of critical-size calvarial defects by baculovirus-engineered MSCs that persistently express growth factors. Biomaterials. 2012;33:3682–3692. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

