Development of the RealTime SARS-CoV-2 quantitative Laboratory Developed Test and correlation with viral culture as a measure of infectivity
- PMID: 34450558
- PMCID: PMC8367731
- DOI: 10.1016/j.jcv.2021.104945
Development of the RealTime SARS-CoV-2 quantitative Laboratory Developed Test and correlation with viral culture as a measure of infectivity
Abstract
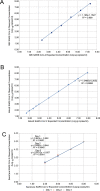
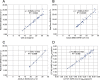
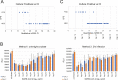
While diagnosis of COVID-19 relies on qualitative molecular testing for the absence or presence of SARS-CoV-2 RNA, quantitative viral load determination for SARS-CoV-2 has many potential applications in antiviral therapy and vaccine trials as well as implications for public health and quarantine guidance. To date, no quantitative SARS-CoV-2 viral load tests have been authorized for clinical use by the FDA. In this study, we modified the FDA emergency use authorized qualitative RealTime SARS-CoV-2 assay into a quantitative SARS-CoV-2 Laboratory Developed Test (LDT) using newly developed Abbott SARS-CoV-2 calibration standards. Both analytical and clinical performance of this SARS-CoV-2 quantitative LDT was evaluated using nasopharyngeal swabs (NPS). We further assessed the correlation between Ct and the ability to culture virus on Vero CCL81 cells. The SARS-CoV-2 quantitative LDT demonstrated high linearity with R2 value of 0.992, high inter- and intra-assay reproducibility across the dynamic range (SDs ± 0.08-0.14 log10 copies/mL for inter-assay reproducibility and ± 0.09 to 0.19 log10 copies/mL for intra-assay reproducibility). Lower limit of detection was determined as 1.90 log10 copies/mL. The highest Ct at which CPE was detected ranged between 28.21-28.49, corresponding to approximately 4.2 log10 copies/mL. Quantitative tests, validated against viral culture capacity, may allow more accurate identification of individuals with and without infectious viral shedding from the respiratory tract.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
WZ, EJDA, FS, and GB do not have conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

