Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study
- PMID: 34450619
- PMCID: PMC8598307
- DOI: 10.1093/jac/dkab318
Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study
Abstract
Objectives: AGILE is a Phase Ib/IIa platform for rapidly evaluating COVID-19 treatments. In this trial (NCT04746183) we evaluated the safety and optimal dose of molnupiravir in participants with early symptomatic infection.
Methods: We undertook a dose-escalating, open-label, randomized-controlled (standard-of-care) Bayesian adaptive Phase I trial at the Royal Liverpool and Broadgreen Clinical Research Facility. Participants (adult outpatients with PCR-confirmed SARS-CoV-2 infection within 5 days of symptom onset) were randomized 2:1 in groups of 6 participants to 300, 600 and 800 mg doses of molnupiravir orally, twice daily for 5 days or control. A dose was judged unsafe if the probability of 30% or greater dose-limiting toxicity (the primary outcome) over controls was 25% or greater. Secondary outcomes included safety, clinical progression, pharmacokinetics and virological responses.
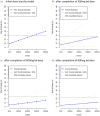
Results: Of 103 participants screened, 18 participants were enrolled between 17 July and 30 October 2020. Molnupiravir was well tolerated at 300, 600 and 800 mg doses with no serious or severe adverse events. Overall, 4 of 4 (100%), 4 of 4 (100%) and 1 of 4 (25%) of the participants receiving 300, 600 and 800 mg molnupiravir, respectively, and 5 of 6 (83%) controls, had at least one adverse event, all of which were mild (≤grade 2). The probability of ≥30% excess toxicity over controls at 800 mg was estimated at 0.9%.
Conclusions: Molnupiravir was safe and well tolerated; a dose of 800 mg twice daily for 5 days was recommended for Phase II evaluation.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Griffiths G, Fitzgerald R, Jaki T. et al. AGILE-ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID-19: a structured summary of a study protocol for a randomised platform trial. Trials 2020; 21: 544. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

