The Toxicity of Universal Dental Adhesives: An In Vitro Study
- PMID: 34451192
- PMCID: PMC8400476
- DOI: 10.3390/polym13162653
The Toxicity of Universal Dental Adhesives: An In Vitro Study
Abstract
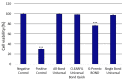
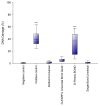
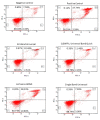
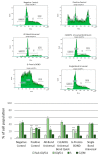
There is no consensus in the literature regarding the potential toxicity of universal dental adhesives (UDA). Being used in close proximity to the pulp, their biocompatibility should be an important factor in dental research. The aim of the present study was to evaluate the biocompatibility of UDA in an in vitro model. The study was performed using a monocyte/macrophage peripheral blood SC cell line (ATCC CRL-9855) on four specific UDA, namely: All-Bond Universal (Bisco); CLEARFIL Universal Bond Quick (Kuraray); G-Premio BOND (GC); Single Bond Universal (3M ESPE). The cytotoxicity of the investigated UDA was measured using the XTT colorimetric assay. The genotoxicity of the analyzed compounds was evaluated using an alkaline version of the comet assay. Furthermore, flow cytometry (FC) apoptosis detection was performed using the FITC Annexin V Apoptosis Detection Kit I. FC cell-cycle arrest assessment was performed using propidium iodide staining. The study observed significant differences in the toxicity of the UDA that were tested, as G-Premio BOND showed significant in vitro toxicity in all of the tests performed, while All-Bond Universal, CLEARFIL Universal Bond Quick and Single Bond Universal did not present any significant toxic effects toward SC cell line. The in vitro toxicity of UDA should be taken into consideration prior to in vivo and clinical studies. The flow cytometry could improve the accuracy of dental materials research and should be incorporated into the standardization criteria.
Keywords: biocompatibility; cytotoxicity; dental materials; flow cytometry; genotoxicity; universal dental adhesives.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures
Similar articles
-
The Cytotoxicity and Genotoxicity of Bioactive Dental Materials.Cells. 2022 Oct 15;11(20):3238. doi: 10.3390/cells11203238. Cells. 2022. PMID: 36291107 Free PMC article.
-
The Cytotoxicity and Genotoxicity of Three Dental Universal Adhesives-An In Vitro Study.Int J Mol Sci. 2020 May 31;21(11):3950. doi: 10.3390/ijms21113950. Int J Mol Sci. 2020. PMID: 32486393 Free PMC article.
-
Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives.J Dent Res. 2016 Apr;95(4):380-7. doi: 10.1177/0022034515623741. Epub 2015 Dec 23. J Dent Res. 2016. PMID: 26701351 Free PMC article.
-
Evaluation of the cytotoxic and genotoxic effects of different universal adhesive systems.J Conserv Dent. 2020 Jul-Aug;23(4):384-389. doi: 10.4103/JCD.JCD_376_20. Epub 2021 Jan 16. J Conserv Dent. 2020. PMID: 33623241 Free PMC article.
-
Effect of double-layer application on the early enamel bond strength of universal adhesives.Clin Oral Investig. 2021 Mar;25(3):907-921. doi: 10.1007/s00784-020-03379-1. Epub 2020 May 30. Clin Oral Investig. 2021. PMID: 32474808
Cited by
-
The Cytotoxicity and Genotoxicity of Bioactive Dental Materials.Cells. 2022 Oct 15;11(20):3238. doi: 10.3390/cells11203238. Cells. 2022. PMID: 36291107 Free PMC article.
-
Effect of Different Application Modalities on the Bonding Performance of Adhesive Systems to Dentin: A Systematic Review and Meta-Analysis.Cells. 2023 Jan 3;12(1):190. doi: 10.3390/cells12010190. Cells. 2023. PMID: 36611983 Free PMC article.
-
A Look Into the Cytotoxicity of Composite Fillings: Friend or Foe?Cureus. 2023 Oct 1;15(10):e46327. doi: 10.7759/cureus.46327. eCollection 2023 Oct. Cureus. 2023. PMID: 37916229 Free PMC article. Review.
-
Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review.Int J Mol Sci. 2023 Dec 21;25(1):152. doi: 10.3390/ijms25010152. Int J Mol Sci. 2023. PMID: 38203323 Free PMC article.
References
-
- Alex G. Universal adhesives: The next evolution in adhesive dentistry? Compend. Contin. Educ. Dent. 2015;36:15–26; quiz 28, 40. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

