PEDOT:PSS-Coated Polybenzimidazole Electroconductive Nanofibers for Biomedical Applications
- PMID: 34451324
- PMCID: PMC8401200
- DOI: 10.3390/polym13162786
PEDOT:PSS-Coated Polybenzimidazole Electroconductive Nanofibers for Biomedical Applications
Abstract
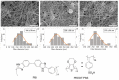
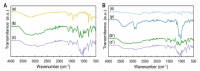
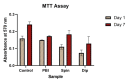
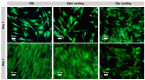
Bioelectricity drives several processes in the human body. The development of new materials that can deliver electrical stimuli is gaining increasing attention in the field of tissue engineering. In this work, novel, highly electrically conductive nanofibers made of poly [2,2'-m-(phenylene)-5,5'-bibenzimidazole] (PBI) have been manufactured by electrospinning and then coated with cross-linked poly (3,4-ethylenedioxythiophene) doped with poly (styrene sulfonic acid) (PEDOT:PSS) by spin coating or dip coating. These scaffolds have been characterized by scanning electron microscopy (SEM) imaging and attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy. The electrical conductivity was measured by the four-probe method at values of 28.3 S·m-1 for spin coated fibers and 147 S·m-1 for dip coated samples, which correspond, respectively, to an increase of about 105 and 106 times in relation to the electrical conductivity of PBI fibers. Human bone marrow-derived mesenchymal stromal cells (hBM-MSCs) cultured on the produced scaffolds for one week showed high viability, typical morphology and proliferative capacity, as demonstrated by calcein fluorescence staining, 4',6-diamidino-2-phenylindole (DAPI)/Phalloidin staining and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide] assay. Therefore, all fiber samples demonstrated biocompatibility. Overall, our findings highlight the great potential of PEDOT:PSS-coated PBI electrospun scaffolds for a wide variety of biomedical applications, including their use as reliable in vitro models to study pathologies and the development of strategies for the regeneration of electroactive tissues or in the design of new electrodes for in vivo electrical stimulation protocols.
Keywords: PBI; PEDOT:PSS; electroconductive; electrospinning; mesenchymal stem cells; nanofibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

