Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers
- PMID: 34451368
- PMCID: PMC8400121
- DOI: 10.3390/polym13162830
Membrane Emulsification Process as a Method for Obtaining Molecularly Imprinted Polymers
Abstract
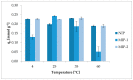
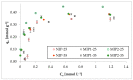
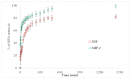
The membrane emulsification process (ME) using a metallic membrane was the first stage for preparing a spherical and monodisperse thermoresponsive molecularly imprinted polymer (TSMIP). In the second step of the preparation, after the ME process, the emulsion of monomers was then polymerized. Additionally, the synthesized TSMIP was fabricated using as a functional monomer N-isopropylacrylamide, which is thermosensitive. This special type of polymer was obtained for the recognition and determination of trace bisphenol A (BPA) in aqueous media. Two types of molecularly imprinted polymers (MIPs) were synthesized using amounts of BPA of 5 wt.% (MIP-2) and 7 wt.% (MIP-1) in the reaction mixtures. Additionally, a non-imprinted polymer (NIP) was also synthesized. Polymer MIP-2 showed thermocontrolled recognition for imprinted molecules and a higher binding capacity than its corresponding non-imprinted polymer and higher than other molecularly imprinted polymer (MIP-1). The best condition for the sorption process was at a temperature of 35 °C, that is, at a temperature close to the phase transition value for poly(N-isopropylacrylamide). Under these conditions, the highest levels of BPA removal from water were achieved and the highest adsorption capacity of MIP-2 was about 0.5 mmol g-1 (about 114.1 mg g-1) and was approximately 20% higher than for MIP-1 and NIP. It was also observed that during the kinetic studies, under these temperature conditions, MIP-2 sorbed BPA faster and with greater efficiency than its non-imprinted analogue.
Keywords: bisphenol A; endocrine disruptors; membrane emulsification process; sorption; thermosensitive molecularly imprinted polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Removal of bisphenol A from aqueous medium using molecularly surface imprinted microbeads.Chemosphere. 2016 May;150:275-284. doi: 10.1016/j.chemosphere.2016.02.040. Epub 2016 Feb 22. Chemosphere. 2016. PMID: 26907596
-
[Preparation of molecularly imprinted polymers-functionalized silica nanoparticles for the separation and recognition of aristolochic acids].Se Pu. 2021 Oct;39(10):1137-1145. doi: 10.3724/SP.J.1123.2021.06024. Se Pu. 2021. PMID: 34505436 Free PMC article. Chinese.
-
Development of Optimal Conditions for Synthesis of Molecularly Imprinted Polymers for Effective Terbium Sorption.Polymers (Basel). 2025 May 19;17(10):1398. doi: 10.3390/polym17101398. Polymers (Basel). 2025. PMID: 40430694 Free PMC article.
-
Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials.Molecules. 2021 Sep 16;26(18):5612. doi: 10.3390/molecules26185612. Molecules. 2021. PMID: 34577083 Free PMC article. Review.
-
[Research progress of molecularly imprinted polymers in separation of chiral drugs by capillary electrochromatography].Se Pu. 2020 Sep 8;38(9):1046-1056. doi: 10.3724/SP.J.1123.2020.03018. Se Pu. 2020. PMID: 34213271 Review. Chinese.
Cited by
-
The Potential of Microbubbles as a Cancer Eradication Theranostic Agent.Pharm Nanotechnol. 2022;10(3):194-209. doi: 10.2174/2211738510666220615154841. Pharm Nanotechnol. 2022. PMID: 35708092 Review.
-
Molecularly Imprinting Microfiltration Membranes Able to Absorb Diethyl Phthalate from Water.Membranes (Basel). 2022 May 8;12(5):503. doi: 10.3390/membranes12050503. Membranes (Basel). 2022. PMID: 35629829 Free PMC article.
References
-
- Piacentini E., Drioli E., Giorno L. Membrane emulsification technology: Twenty-five years of inventions and research through patent survey. J. Membr. Sci. 2014;468:410–422. doi: 10.1016/j.memsci.2014.05.059. - DOI
-
- Wolska J., Bryjak M. Preparation of poly(styrene-co-divinylbenzene) microspheres by membrane emulsification. Desalination. 2009;241:331–336. doi: 10.1016/j.desal.2008.01.070. - DOI
-
- Arkoumanis P.G., Norton I.T., Spyropoulos F. Pickering particle and emulsifier co-stabilised emulsions produced via rotating membrane emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2019;568:481–492. doi: 10.1016/j.colsurfa.2019.02.036. - DOI
-
- Joscelyne S.M., Trägårdh G. Membrane emulsification—A literature review. J. Membr. Sci. 2000;169:107–117. doi: 10.1016/S0376-7388(99)00334-8. - DOI
-
- Wolska J., Bryjak M. Molecularly Imprinted Polymers for Water Polishing. In: Dragan E.S., editor. Advanced Separations by Specialized Sorbents. Chromatographic Science Series. Vol. 108. CRC Press Taylor and Francis Group; Boca Raton, FL, USA: New York, NY, USA: London, UK: 2015. pp. 195–208.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

