Staphylococcus aureus Synergized with Candida albicans to Increase the Pathogenesis and Drug Resistance in Cutaneous Abscess and Peritonitis Murine Models
- PMID: 34451500
- PMCID: PMC8398722
- DOI: 10.3390/pathogens10081036
Staphylococcus aureus Synergized with Candida albicans to Increase the Pathogenesis and Drug Resistance in Cutaneous Abscess and Peritonitis Murine Models
Abstract
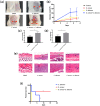
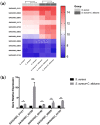
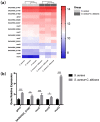
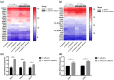
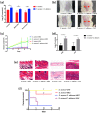
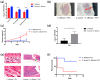
The mixed species of Staphylococcus aureus and Candida albicans can cause infections on skin, mucosa or bloodstream; however, mechanisms of their cross-kingdom interactions related to pathogenesis and drug resistance are still not clear. Here an increase of S. aureus proliferation and biofilm formation was observed in S. aureus and C. albicans dual-species culture, and the synergistic pathogenic effect was then confirmed in both local (cutaneous abscess) and systemic infection (peritonitis) murine models. According to the transcriptome analysis of the dual-species culture, virulence factors of S. aureus were significantly upregulated. Surprisingly, the beta-lactams and vancomycin-resistant genes in S. aureus as well as azole-resistant genes in C. albicans were also significantly increased. The synergistic effects on drug resistance to both antibacterial and antifungal agents were further proved both in vitro and in cutaneous abscess and peritonitis murine models treated by methicillin, vancomycin and fluconazole. The synergistic interactions between S. aureus and C. albicans on pathogenesis and drug resistance highlight the importance of targeting the microbial interactions in polyspecies-associated infections.
Keywords: Candida albicans; Staphylococcus aureus; drug resistance; synergistic effect; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida sp.J Appl Microbiol. 2020 Jan;128(1):88-101. doi: 10.1111/jam.14443. Epub 2019 Nov 19. J Appl Microbiol. 2020. PMID: 31509623
-
[Analysis of distribution and drug resistance of pathogens from the wounds of 1 310 thermal burn patients].Zhonghua Shao Shang Za Zhi. 2018 Nov 20;34(11):802-808. doi: 10.3760/cma.j.issn.1009-2587.2018.11.016. Zhonghua Shao Shang Za Zhi. 2018. PMID: 30481922 Chinese.
-
Candida albicans Augments Staphylococcus aureus Virulence by Engaging the Staphylococcal agr Quorum Sensing System.mBio. 2019 Jun 4;10(3):e00910-19. doi: 10.1128/mBio.00910-19. mBio. 2019. PMID: 31164467 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Antifungal drug resistance in pathogenic fungi.Med Mycol. 1998;36 Suppl 1:119-28. Med Mycol. 1998. PMID: 9988500 Review.
Cited by
-
Co-colonization of methicillin-resistant Staphylococcus aureus and Candida spp. in children with malignancies.AMB Express. 2024 Feb 13;14(1):22. doi: 10.1186/s13568-024-01667-7. AMB Express. 2024. PMID: 38351284 Free PMC article.
-
Lycosin-II Exhibits Antifungal Activity and Inhibits Dual-Species Biofilm by Candida albicans and Staphylococcus aureus.J Fungi (Basel). 2022 Aug 24;8(9):901. doi: 10.3390/jof8090901. J Fungi (Basel). 2022. PMID: 36135626 Free PMC article.
-
Inhibition of Mixed Biofilms of Candida albicans and Staphylococcus aureus by β-Caryophyllene-Gold Nanoparticles.Antibiotics (Basel). 2023 Apr 7;12(4):726. doi: 10.3390/antibiotics12040726. Antibiotics (Basel). 2023. PMID: 37107087 Free PMC article.
-
Enhanced Virulence of Candida albicans by Staphylococcus aureus: Evidence in Clinical Bloodstream Infections and Infected Zebrafish Embryos.J Fungi (Basel). 2021 Dec 20;7(12):1099. doi: 10.3390/jof7121099. J Fungi (Basel). 2021. PMID: 34947081 Free PMC article.
-
A customizable and defined medium supporting culturing of Candida albicans, Staphylococcus aureus, and human oral epithelial cells.Appl Environ Microbiol. 2024 Aug 21;90(8):e0036024. doi: 10.1128/aem.00360-24. Epub 2024 Jul 29. Appl Environ Microbiol. 2024. PMID: 39072650 Free PMC article.
References
-
- Armbruster C.E., Hong W., Pang B., Weimer K.E., Juneau R.A., Turner J., Swords W.E. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio. 2010;1:e00102-10. doi: 10.1128/mBio.00102-10. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

