Agrobacterium- Mediated Genetic Transformation of Taiwanese Isolates of Lemna aequinoctialis
- PMID: 34451621
- PMCID: PMC8401387
- DOI: 10.3390/plants10081576
Agrobacterium- Mediated Genetic Transformation of Taiwanese Isolates of Lemna aequinoctialis
Abstract
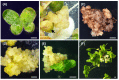
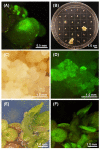
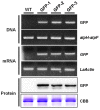
Duckweed (Lemna aequinoctialis) is one of the smallest flowering plants in the world. Due to its high reproduction rate and biomass, duckweeds are used as biofactors and feedstuff additives for livestock. It is also an ideal system for basic biological research and various practical applications. In this study, we attempt to establish a micropropagation technique and Agrobacterium-mediated transformation in L. aequinoctialis. The plant-growth regulator type and concentration and Agrobacterium-mediated transformation were evaluated for their effects on duckweed callus induction, proliferation, regeneration, and gene transformation efficiency. Calli were successfully induced from 100% of explants on Murashige and Skoog (MS) medium containing 25.0 μM 2,4-dichlorophenoxyacetic acid (2,4-D) and 2.0 μM thidiazuron (TDZ). MS medium containing 4.5 μM 2,4-D and 2.0 μM TDZ supported the long-lasting growth of calli. Fronds regenerated from 100% of calli on Schenk and Hildebrandt (SH) medium containing 1.0 μM 6-benzyladenine (6-BA). We also determined that 200 μM acetosyringone in the cocultivation medium for 1 day in the dark was crucial for transformation efficiency (up to 3 ± 1%). Additionally, we propose that both techniques will facilitate efficient high-throughput genetic manipulation in Lemnaceae.
Keywords: Agrobacterium tumefaciens; duckweed; tissue culture; transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Appenroth K., Borisjuk N., Lam E. Telling duckweed apart: Genotyping technologies for the Lemnaceae. Chin. J. Appl. Environ. Biol. 2013;19:1–10.
-
- Landolt E. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae) Volume 1 Stiftung Rübel; Zurich, Switzerland: 1986. The family of Lemnaceae-a monographic study. Veröffentlichungen des Geobotanischen Institutes der ETH.
-
- Sree K.S., Bog M., Appenroth K.J. Taxonomy of duckweeds (Lemnaceae), potential new crop plants. Emir. J. Food Agric. 2016;28:291–302. doi: 10.9755/ejfa.2016-01-038. - DOI
-
- Stomp A.M. The duckweeds: A valuable plant for biomanufacturing. Biotechnol. Annu. Rev. 2005;11:69–99. - PubMed
-
- Yang J., Li G., Hu S., Bishopp A., Heenatigala P., Kumar S., Duan P., Yao L., Hou H. A protocol for efficient callus induction and stable transformation of Spirodela polyrhiza (L.) Schleiden using Agrobacterium tumefaciens. Aquat. Bot. 2018;151:80–86. doi: 10.1016/j.aquabot.2018.08.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

