Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles
- PMID: 34451715
- PMCID: PMC8400420
- DOI: 10.3390/plants10081671
Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles
Abstract
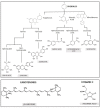
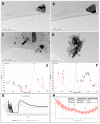
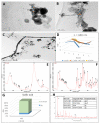
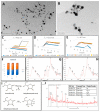
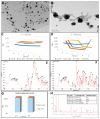
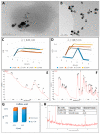
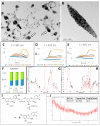
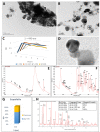
The main antioxidants present in plant extracts-quercetin, β-carotene, gallic acid, ascorbic acid, hydroxybenzoic acid, caffeic acid, catechin and scopoletin-are able to synthesize silver nanoparticles when reacting with a Ag NO3 solution. The UV-visible absorption spectrum recorded with most of the antioxidants shows the characteristic surface plasmon resonance band of silver nanoparticles. Nanoparticles synthesised with ascorbic, hydroxybenzoic, caffeic, and gallic acids and scopoletin are spherical. Nanoparticles synthesised with quercetin are grouped together to form micellar structures. Nanoparticles synthesised by β-carotene, were triangular and polyhedral forms with truncated corners. Pentagonal nanoparticles were synthesized with catechin. We used Fourier-transform infrared spectroscopy to check that the biomolecules coat the synthesised silver nanoparticles. X-ray powder diffractograms showed the presence of silver, AgO, Ag2O, Ag3O4 and Ag2O3. Rod-like structures were obtained with quercetin and gallic acid and cookie-like structures in the nanoparticles obtained with scopoletin, as a consequence of their reactivity with cyanide. This analysis explained the role played by the various agents responsible for the bio-reduction triggered by nanoparticle synthesis in their shape, size and activity. This will facilitate targeted synthesis and the application of biotechnological techniques to optimise the green synthesis of nanoparticles.
Keywords: cyanide; micellar structures; plant non-enzymatic antioxidants; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis, Characteristation and Biological Activity of Silver Nanoparticles Generated Using the Leaf and Stembark Extract of Combretum erythrophyllum.Anticancer Agents Med Chem. 2023;23(13):1545-1566. doi: 10.2174/1871520623666230417112903. Anticancer Agents Med Chem. 2023. PMID: 37073157
-
Green Synthesis, Characterization, Enzyme Inhibition, Antimicrobial Potential, and Cytotoxic Activity of Plant Mediated Silver Nanoparticle Using Ricinus communis Leaf and Root Extracts.Biomolecules. 2021 Feb 2;11(2):206. doi: 10.3390/biom11020206. Biomolecules. 2021. PMID: 33540690 Free PMC article.
-
Sunlight-Mediated Green Synthesis of Silver Nanoparticles Using the Berries of Ribes rubrum (Red Currants): Characterisation and Evaluation of Their Antifungal and Antibacterial Activities.Molecules. 2022 Mar 28;27(7):2186. doi: 10.3390/molecules27072186. Molecules. 2022. PMID: 35408589 Free PMC article.
-
Bioactive compound loaded stable silver nanoparticle synthesis from microwave irradiated aqueous extracellular leaf extracts of Naringi crenulata and its wound healing activity in experimental rat model.Acta Trop. 2014 Jul;135:55-61. doi: 10.1016/j.actatropica.2014.03.009. Epub 2014 Mar 26. Acta Trop. 2014. PMID: 24681224
-
Green Synthesis and Characterisation of Silver Nanoparticles Using Cassia tora Seed Extract and Investigation of Antibacterial Potential.Appl Biochem Biotechnol. 2022 Jan;194(1):464-478. doi: 10.1007/s12010-021-03651-4. Epub 2021 Oct 6. Appl Biochem Biotechnol. 2022. PMID: 34611854
Cited by
-
Phytosynthesis of Silver Nanoparticles Using Mansoa alliacea (Lam.) A.H. Gentry (Bignoniaceae) Leaf Extract: Characterization and Their Biological Activities.Pharmaceutics. 2024 Sep 25;16(10):1247. doi: 10.3390/pharmaceutics16101247. Pharmaceutics. 2024. PMID: 39458579 Free PMC article.
-
Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes.Materials (Basel). 2022 Jan 20;15(3):775. doi: 10.3390/ma15030775. Materials (Basel). 2022. PMID: 35160720 Free PMC article.
-
Critical Evaluation of Green Synthesized Silver Nanoparticles-Kaempferol for Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus.Int J Nanomedicine. 2024 Feb 8;19:1339-1350. doi: 10.2147/IJN.S431499. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38348172 Free PMC article.
-
Formulation of Garlic Essential Oil-assisted Silver Nanoparticles and Mechanistic Evaluation of their Antimicrobial Activity against a Spectrum of Pathogenic Microorganisms.Curr Top Med Chem. 2024;24(22):2000-2012. doi: 10.2174/0115680266322180240712055727. Curr Top Med Chem. 2024. PMID: 39092647 Free PMC article.
-
Algal-Derived Synthesis of Silver Nanoparticles Using the Unicellular ulvophyte sp. MBIC10591: Optimisation, Characterisation, and Biological Activities.Molecules. 2022 Dec 29;28(1):279. doi: 10.3390/molecules28010279. Molecules. 2022. PMID: 36615473 Free PMC article.
References
-
- European Commision. [(accessed on 8 May 2021)];2018 Available online: https://ec.europa.eu/programmes/horizon2020/en/h2020-section/nanotechnol....
-
- Raveendran P., Fu J., Wallen S.L. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006;8:34–38. doi: 10.1039/B512540E. - DOI
-
- Saratale R.G., Saratale G.D., Shin H.S., Jacob J.M., Pugazhendhi A., Bhaisare M., Kumar G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018;25:10164–10183. doi: 10.1007/s11356-017-9912-6. - DOI - PubMed
-
- Rotello V.M., editor. Nanoparticles: Building Blocks for Nanotechnology. Springer Science & Business Media; Berlin/Heidelberg, Germany: 2004.
-
- Srikar S.K., Giri D.D., Pal D.B., Mishra P.K., Upadhyay S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016;6:34–56. doi: 10.4236/gsc.2016.61004. - DOI
LinkOut - more resources
Full Text Sources

