Micrografting Provides Evidence for Systemic Regulation of Sulfur Metabolism between Shoot and Root
- PMID: 34451773
- PMCID: PMC8402062
- DOI: 10.3390/plants10081729
Micrografting Provides Evidence for Systemic Regulation of Sulfur Metabolism between Shoot and Root
Abstract
The uptake of sulfate by roots and its reductive assimilation mainly in the leaves are not only essential for plant growth and development but also for defense responses against biotic and abiotic stresses. The latter functions result in stimulus-induced fluctuations of sulfur demand at the cellular level. However, the maintenance and acclimation of sulfur homeostasis at local and systemic levels is not fully understood. Previous research mostly focused on signaling in response to external sulfate supply to roots. Here we apply micrografting of Arabidopsis wildtype knock-down sir1-1 mutant plants that suffer from an internally lowered reductive sulfur assimilation and a concomitant slow growth phenotype. Homografts of wildtype and sir1-1 confirm the hallmarks of non-grafted sir1-1 mutants, displaying substantial induction of sulfate transporter genes in roots and sulfate accumulation in shoots. Heterografts of wildtype scions and sir1-1 rootstocks and vice versa, respectively, demonstrate a dominant role of the shoot over the root with respect to sulfur-related gene expression, sulfate accumulation and organic sulfur metabolites, including the regulatory compound O-acetylserine. The results provide evidence for demand-driven control of the shoot over the sulfate uptake system of roots under sulfur-sufficient conditions, allowing sulfur uptake and transport to the shoot for dynamic responses.
Keywords: O-acetylserine; grafting; organ communication; sulfate transporter; sulfite reductase; sulfur homeostasis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
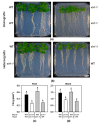
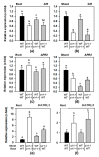
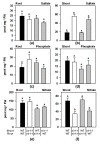
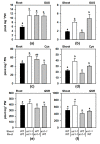
Similar articles
-
Local and systemic regulation of sulfur homeostasis in roots of Arabidopsis thaliana.Plant J. 2012 Nov;72(4):625-35. doi: 10.1111/j.1365-313X.2012.05105.x. Epub 2012 Sep 20. Plant J. 2012. PMID: 22775482
-
Effects of Cadmium Treatment on the Uptake and Translocation of Sulfate in Arabidopsis thaliana.Plant Cell Physiol. 2016 Nov;57(11):2353-2366. doi: 10.1093/pcp/pcw156. Epub 2016 Sep 1. Plant Cell Physiol. 2016. PMID: 27590710
-
Local and Systemic Response to Heterogeneous Sulfate Resupply after Sulfur Deficiency in Rice.Int J Mol Sci. 2022 May 31;23(11):6203. doi: 10.3390/ijms23116203. Int J Mol Sci. 2022. PMID: 35682882 Free PMC article.
-
Manipulation of thiol contents in plants.Amino Acids. 2001;20(3):291-9. doi: 10.1007/s007260170045. Amino Acids. 2001. PMID: 11354605 Review.
-
Thiol redox-regulation for efficient adjustment of sulfur metabolism in acclimation to abiotic stress.J Exp Bot. 2019 Aug 19;70(16):4223-4236. doi: 10.1093/jxb/erz118. J Exp Bot. 2019. PMID: 30868161 Review.
Cited by
-
Discriminative Long-Distance Transport of Selenate and Selenite Triggers Glutathione Oxidation in Specific Subcellular Compartments of Root and Shoot Cells in Arabidopsis.Front Plant Sci. 2022 Jun 24;13:894479. doi: 10.3389/fpls.2022.894479. eCollection 2022. Front Plant Sci. 2022. PMID: 35812960 Free PMC article.
-
Quantitative Trait Loci Mappings for the Sulfur Utilization Efficiency-Related Traits at the Seedling Stage of Wheat.Genes (Basel). 2024 Nov 29;15(12):1550. doi: 10.3390/genes15121550. Genes (Basel). 2024. PMID: 39766816 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

