Induction of Conjugation and Zygospore Cell Wall Characteristics in the Alpine Spirogyra mirabilis (Zygnematophyceae, Charophyta): Advantage under Climate Change Scenarios?
- PMID: 34451785
- PMCID: PMC8402014
- DOI: 10.3390/plants10081740
Induction of Conjugation and Zygospore Cell Wall Characteristics in the Alpine Spirogyra mirabilis (Zygnematophyceae, Charophyta): Advantage under Climate Change Scenarios?
Abstract
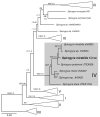
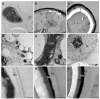
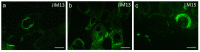
Extreme environments, such as alpine habitats at high elevation, are increasingly exposed to man-made climate change. Zygnematophyceae thriving in these regions possess a special means of sexual reproduction, termed conjugation, leading to the formation of resistant zygospores. A field sample of Spirogyra with numerous conjugating stages was isolated and characterized by molecular phylogeny. We successfully induced sexual reproduction under laboratory conditions by a transfer to artificial pond water and increasing the light intensity to 184 µmol photons m-2 s-1. This, however was only possible in early spring, suggesting that the isolated cultures had an internal rhythm. The reproductive morphology was characterized by light- and transmission electron microscopy, and the latter allowed the detection of distinctly oriented microfibrils in the exo- and endospore, and an electron-dense mesospore. Glycan microarray profiling showed that Spirogyra cell walls are rich in major pectic and hemicellulosic polysaccharides, and immuno-fluorescence allowed the detection of arabinogalactan proteins (AGPs) and xyloglucan in the zygospore cell walls. Confocal RAMAN spectroscopy detected complex aromatic compounds, similar in their spectral signature to that of Lycopodium spores. These data support the idea that sexual reproduction in Zygnematophyceae, the sister lineage to land plants, might have played an important role in the process of terrestrialization.
Keywords: Spirogyra; alpine region; cell wall; conjugation; sexual reproduction; streptophyte; zygospore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

