Transcranial Focal Electrical Stimulation Modifies Biogenic Amines' Alterations Induced by 6-Hydroxydopamine in Rat Brain
- PMID: 34451804
- PMCID: PMC8401891
- DOI: 10.3390/ph14080706
Transcranial Focal Electrical Stimulation Modifies Biogenic Amines' Alterations Induced by 6-Hydroxydopamine in Rat Brain
Abstract
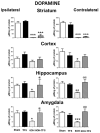
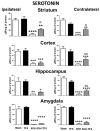
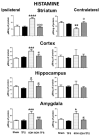
Transcranial focal stimulation (TFS) is a non-invasive neuromodulation strategy with neuroprotective effects. On the other hand, 6-hidroxidopamine (6-OHDA) induces neurodegeneration of the nigrostriatal system producing modifications in the dopaminergic, serotoninergic, and histaminergic systems. The present study was conducted to test whether repetitive application of TFS avoids the biogenic amines' changes induced by the intrastriatal injection of 6-OHDA. Experiments were designed to determine the tissue content of dopamine, serotonin, and histamine in the brain of animals injected with 6-OHDA and then receiving daily TFS for 21 days. Tissue content of biogenic amines was evaluated in the cerebral cortex, hippocampus, amygdala, and striatum, ipsi- and contralateral to the side of 6-OHDA injection. Results obtained were compared to animals with 6-OHDA, TFS alone, and a Sham group. The present study revealed that TFS did not avoid the changes in the tissue content of dopamine in striatum. However, TFS was able to avoid several of the changes induced by 6-OHDA in the tissue content of dopamine, serotonin, and histamine in the different brain areas evaluated. Interestingly, TFS alone did not induce significant changes in the different brain areas evaluated. The present study showed that repetitive TFS avoids the biogenic amines' changes induced by 6-OHDA. TFS can represent a new therapeutic strategy to avoid the neurotoxicity induced by 6-OHDA.
Keywords: 6-hidroxidopamine; dopamine; histamine; non-invasive neuromodulation; serotonin; transcranial focal stimulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Multiple Administration of Endogenous Amines TIQ and 1MeTIQ Protects Against a 6-OHDA-Induced Essential Fall of Dopamine Release in the Rat Striatum: In Vivo Microdialysis Study.Neurotox Res. 2018 Apr;33(3):523-531. doi: 10.1007/s12640-017-9824-8. Epub 2017 Oct 26. Neurotox Res. 2018. PMID: 29076060 Free PMC article.
-
Selective lesions by manganese and extensive damage by iron after injection into rat striatum or hippocampus.J Neurochem. 1994 Jan;62(1):205-16. doi: 10.1046/j.1471-4159.1994.62010205.x. J Neurochem. 1994. PMID: 7505311
-
Alterations of the nigrostriatal pathway in a 6-OHDA rat model of Parkinson's disease evaluated with multimodal MRI.PLoS One. 2018 Sep 6;13(9):e0202597. doi: 10.1371/journal.pone.0202597. eCollection 2018. PLoS One. 2018. PMID: 30188909 Free PMC article.
-
Noninvasive transcranial focal stimulation affects the convulsive seizure-induced P-glycoprotein expression and function in rats.Epilepsy Behav. 2021 Feb;115:107659. doi: 10.1016/j.yebeh.2020.107659. Epub 2020 Dec 15. Epilepsy Behav. 2021. PMID: 33334719
-
R-(-)-deprenyl (Selegiline, Movergan) facilitates the activity of the nigrostriatal dopaminergic neuron.J Neural Transm Suppl. 1987;25:45-66. J Neural Transm Suppl. 1987. PMID: 2828537 Review.

