Antibodies Enhance the Suppressive Activity of Extracellular Vesicles in Mouse Delayed-Type Hypersensitivity
- PMID: 34451831
- PMCID: PMC8398949
- DOI: 10.3390/ph14080734
Antibodies Enhance the Suppressive Activity of Extracellular Vesicles in Mouse Delayed-Type Hypersensitivity
Abstract
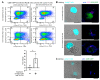
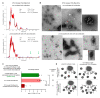
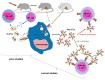
Previously, we showed that mouse delayed-type hypersensitivity (DTH) can be antigen-specifically downregulated by suppressor T cell-derived miRNA-150 carried by extracellular vesicles (EVs) that target antigen-presenting macrophages. However, the exact mechanism of the suppressive action of miRNA-150-targeted macrophages on effector T cells remained unclear, and our current studies aimed to investigate it. By employing the DTH mouse model, we showed that effector T cells were inhibited by macrophage-released EVs in a miRNA-150-dependent manner. This effect was enhanced by the pre-incubation of EVs with antigen-specific antibodies. Their specific binding to MHC class II-expressing EVs was proved in flow cytometry and ELISA-based experiments. Furthermore, by the use of nanoparticle tracking analysis and transmission electron microscopy, we found that the incubation of macrophage-released EVs with antigen-specific antibodies resulted in EVs' aggregation, which significantly enhanced their suppressive activity in vivo. Nowadays, it is increasingly evident that EVs play an exceptional role in intercellular communication and selective cargo transfer, and thus are considered promising candidates for therapeutic usage. However, EVs appear to be less effective than their parental cells. In this context, our current studies provide evidence that antigen-specific antibodies can be easily used for increasing EVs' biological activity, which has great therapeutic potential.
Keywords: antigen-presenting cells; antigen-specific T cell suppression; contact hypersensitivity; delayed-type hypersensitivity; extracellular vesicles; immune tolerance; intercellular communication; macrophages; miRNA-150; therapeutic activity of exosomes.
Conflict of interest statement
K.N. and K.B. declare that they are inventors in a patent application number P.435582 submitted by Jagiellonian University, Krakow, Poland for the method of generating the antibody-aggregated macrophage EVs. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Orally Administered Exosomes Suppress Mouse Delayed-Type Hypersensitivity by Delivering miRNA-150 to Antigen-Primed Macrophage APC Targeted by Exosome-Surface Anti-Peptide Antibody Light Chains.Int J Mol Sci. 2020 Aug 2;21(15):5540. doi: 10.3390/ijms21155540. Int J Mol Sci. 2020. PMID: 32748889 Free PMC article.
-
Syngeneic red blood cell-induced extracellular vesicles suppress delayed-type hypersensitivity to self-antigens in mice.Clin Exp Allergy. 2019 Nov;49(11):1487-1499. doi: 10.1111/cea.13475. Epub 2019 Aug 26. Clin Exp Allergy. 2019. PMID: 31365154 Free PMC article.
-
Delayed-Type Hypersensitivity Underlying Casein Allergy Is Suppressed by Extracellular Vesicles Carrying miRNA-150.Nutrients. 2019 Apr 23;11(4):907. doi: 10.3390/nu11040907. Nutrients. 2019. PMID: 31018604 Free PMC article.
-
Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells.Annu Rev Immunol. 2018 Apr 26;36:435-459. doi: 10.1146/annurev-immunol-041015-055700. Epub 2018 Jan 31. Annu Rev Immunol. 2018. PMID: 29400984 Review.
-
The Methods of Choice for Extracellular Vesicles (EVs) Characterization.Int J Mol Sci. 2017 May 29;18(6):1153. doi: 10.3390/ijms18061153. Int J Mol Sci. 2017. PMID: 28555055 Free PMC article. Review.
Cited by
-
Increasing the Therapeutic Efficacy of Extracellular Vesicles From the Antigen-Specific Antibody and Light Chain Perspective.Front Cell Dev Biol. 2021 Nov 24;9:790722. doi: 10.3389/fcell.2021.790722. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901032 Free PMC article.
-
Exosome Carrier Effects; Resistance to Digestion in Phagolysosomes May Assist Transfers to Targeted Cells; II Transfers of miRNAs Are Better Analyzed via Systems Approach as They Do Not Fit Conventional Reductionist Stoichiometric Concepts.Int J Mol Sci. 2022 May 31;23(11):6192. doi: 10.3390/ijms23116192. Int J Mol Sci. 2022. PMID: 35682875 Free PMC article. Review.
-
Biodelivery of therapeutic extracellular vesicles: should mononuclear phagocytes always be feared?Front Cell Dev Biol. 2023 Jul 5;11:1211833. doi: 10.3389/fcell.2023.1211833. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37476156 Free PMC article. Review.
-
Extracellular Vesicles-Oral Therapeutics of the Future.Int J Mol Sci. 2022 Jul 7;23(14):7554. doi: 10.3390/ijms23147554. Int J Mol Sci. 2022. PMID: 35886902 Free PMC article. Review.
-
Macrophage Functions in Psoriasis: Lessons from Mouse Models.Int J Mol Sci. 2024 May 13;25(10):5306. doi: 10.3390/ijms25105306. Int J Mol Sci. 2024. PMID: 38791342 Free PMC article. Review.
References
-
- Bryniarski K., Ptak W., Jayakumar A., Püllmann K., Caplan M.J., Chairoungdua A., Lu J., Adams B.D., Sikora E., Nazimek K., et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 2013;132:170–181.e9. doi: 10.1016/j.jaci.2013.04.048. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

