Synthesis and Biological Evaluation of a Radiolabeled PET (Positron Emission Tomography) Probe for Visualization of In Vivo α-Fucosidase Expression
- PMID: 34451843
- PMCID: PMC8402005
- DOI: 10.3390/ph14080745
Synthesis and Biological Evaluation of a Radiolabeled PET (Positron Emission Tomography) Probe for Visualization of In Vivo α-Fucosidase Expression
Abstract
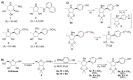
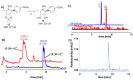
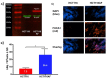
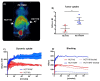
The acidic hydrolase α-fucosidase (AF) is a biomarker for maladies such as cancer and inflammation. The most advanced probes for α-fucosidase are unfortunately constrained to ex vivo or in vitro applications. The in vivo detection and quantification of AF using positron emission tomography would allow for better discovery and diagnosis of disease as well as provide better understanding of disease progression. We synthesized, characterized, and evaluated a radiolabeled small molecule inhibitor of AF based on a known molecule. The radiosynthesis involved the 11C methylation of a phenoxide, which was generated in situ by ultrasonification of the precursor with sodium hydride. The tracer was produced with a decay corrected yield of 41.7 ± 16.5% and had a molar activity of 65.4 ± 30.3 GBq/μmol. The tracer was shown to be stable in mouse serum at 60 min. To test the new tracer, HCT116 colorectal carcinoma cells were engineered to overexpress human AF. In vitro evaluation revealed 3.5-fold higher uptake in HCT116AF cells compared to HCT116 controls (26.4 ± 7.8 vs. 7.5 ± 1.0 kBq/106 cells). Static PET scans 50 min post injection revealed 2.5-fold higher tracer uptake in the HCT116AF tumors (3.0 ± 0.8%ID/cc (n = 6)) compared with the controls (1.2 ± 0.8 (n = 5)). Dynamic scans showed higher uptake in the HCT116AF tumors at all time-points (n = 2). Ex vivo analysis of the tumors, utilizing fluorescent DDK2 antibodies, confirmed the expression of human AF in the HCT116AF xenografts. We have developed a novel PET tracer to image AF in vivo and will now apply this to relevant disease models.
Keywords: 11C; PET tracer; cancer; inflammation; α-fucosidase.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Evaluation of N-[(11)C]methyl-AMD3465 as a PET tracer for imaging of CXCR4 receptor expression in a C6 glioma tumor model.Mol Pharm. 2014 Nov 3;11(11):3810-7. doi: 10.1021/mp500398r. Epub 2014 Aug 18. Mol Pharm. 2014. PMID: 25094028
-
Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor alphavbeta3-integrin expression.J Nucl Med. 2004 Oct;45(10):1776-83. J Nucl Med. 2004. PMID: 15471848
-
One-step 18F-fluorination of smart positron emission tomography tracer for sensing furin activity in tumors.Nucl Med Biol. 2020 Mar-Apr;82-83:72-79. doi: 10.1016/j.nucmedbio.2020.02.010. Epub 2020 Feb 20. Nucl Med Biol. 2020. PMID: 32109829
-
Efficiency gains in tracer identification for nuclear imaging: can in vivo LC-MS/MS evaluation of small molecules screen for successful PET tracers?ACS Chem Neurosci. 2014 Dec 17;5(12):1154-63. doi: 10.1021/cn500073j. Epub 2014 Oct 13. ACS Chem Neurosci. 2014. PMID: 25247893 Review.
-
Use of PET in neuroendocrine tumors. In vivo applications and in vitro studies.Q J Nucl Med. 2000 Mar;44(1):68-76. Q J Nucl Med. 2000. PMID: 10932603 Review.
References
-
- Sobkowicz A.D., Gallagher M.E., Reid C.J., Crean D., Carrington S.D., Irwin J.A. Modulation of Expression in BEAS-2B Airway Epithelial Cells of α-l-Fucosidase A1 and A2 by Th1 and Th2 Cytokines, and Overexpression of α-l-Fucosidase 2. Mol. Cell Biochem. 2014;390:101–113. doi: 10.1007/s11010-014-1961-2. - DOI - PubMed
-
- Cheng T., Tu S., Chen L.-C., Chen M.-Y., Chen W.-Y., Lin Y.-K., Ho C.-T., Lin S.-Y., Wu C.-H., Ho Y.-S. Down-Regulation of α-L-Fucosidase 1 Expression Confers Inferior Survival for Triple-Negative Breast Cancer Patients by Modulating the Glycosylation Status of the Tumor Cell Surface. Oncotarget. 2015;6:21283–21300. doi: 10.18632/oncotarget.4238. - DOI - PMC - PubMed
-
- Otero-Estévez O., Martínez-Fernández M., Vázquez-Iglesias L., Páez de la Cadena M., Rodríguez-Berrocal F.J., Martínez-Zorzano V.S. Decreased Expression of Alpha-L-Fucosidase gene FUCA1 in Human Colorectal Tumors. Int. J. Mol. Sci. 2013;14:16986–16998. doi: 10.3390/ijms140816986. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

