Synthesis and Biological Activities of Pyrazino[1,2- a]indole and Pyrazino[1,2- a]indol-1-one Derivatives
- PMID: 34451876
- PMCID: PMC8399128
- DOI: 10.3390/ph14080779
Synthesis and Biological Activities of Pyrazino[1,2- a]indole and Pyrazino[1,2- a]indol-1-one Derivatives
Abstract
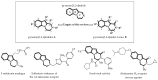
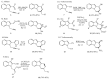
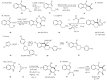
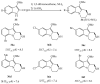
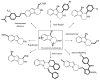
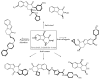
This review concerns the synthesis and biological activities of pyrazino[1,2-a]indoles and pyrazino[1,2-a]indol-1-ones reported since 1997 and the discovery of biological activity of pyrazinoindole derivatives. In the first part, we first presented the synthetic routes that have been reported from a methodological point of view to access the pyrazinoindole unit according to cyclization reactions using or not using metal catalysts. Then, syntheses and neuropsychiatric, auto-immune, anti-infectious and anti-cancer properties of pyrazinoindoles were detailed. In the second part, we first reported the main accesses to pyrazinoindol-1-one substrates according to Michael reactions, metal-catalyzed and metal-free cyclization reactions. The syntheses and anti-cancer, anti-infectious, anti-allergenic and neuropsychiatric properties of pyrazinoindolones were next described and discussed.
Keywords: biological activity; catalysis; cyclization; pyrazinoindole; pyrazinoindolone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

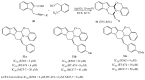
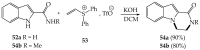
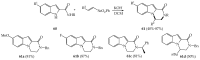
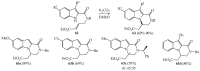
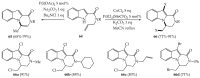
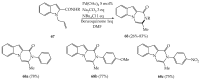
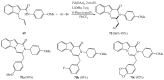
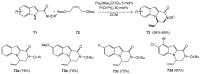
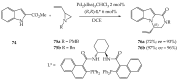

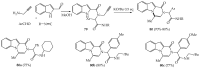
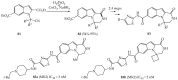
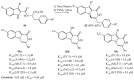
References
-
- Singh A., Mahapatra S., Sewariya S., Singh N., Singh S., Kumar Y., Bandichhor R., Chandra R. A mini-review on the synthesis of pyrazinoindole: Recent progress and perspectives. Mini Rev. Org. Chem. 2021;18:504–514. doi: 10.2174/1570193X17999200806151209. - DOI
-
- Sokolova E.A., Festa A.A. Synthesis of pyrazino[1,2-a] indoles and indolo [1,2-a] quinoxalines (microreview) Chem. Heterocycl. Comp. 2016;52:219–221. doi: 10.1007/s10593-016-1866-y. - DOI
-
- Markl C., Attia M.I., Julius J., Sehti S., Witt-Enderby P.A., Zlotos D.P. Synthesis and pharmacological evaluation of 1,2,3,4-tetrahydropyrazino[1,2-a] indole and 2-[(phenylmethylamino)methyl]-1H-indole analogues as novel melatoninergic ligands. Bioorg. Med. Chem. 2009;17:4583–4594. doi: 10.1016/j.bmc.2009.04.068. - DOI - PubMed
-
- Romagnoli R., Baraldi P.G., Carrion M.D., Cara C.L., Salvador M.K., Preti D., Tabrizi M.A., Moorman A.R., Vincenzi F., Borea P.A., et al. Synthesis and biological effects of novel 2-amino-3-(4-chlorobenzoyl)-4-substituted thiophenes as allosteric enhancers of the A1 adenosine receptor. Eur. J. Med. Chem. 2013;67:409–427. doi: 10.1016/j.ejmech.2013.07.002. - DOI - PubMed
-
- Kounde C.S., Yeo H.Q., Wang Q.Y., Wan K.F., Dong H., Karuna R., Dix I., Wagner T., Zou B., Simon O. Discovery of 2-oxopiperazine dengue inhibitors by scaffold morphing of a phenotypic high-throughput screening hit. Bioorg. Med. Chem. Lett. 2017;27:1385–1389. doi: 10.1016/j.bmcl.2017.02.005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

