A High-Content Screen for the Identification of Plant Extracts with Insulin Secretion-Modulating Activity
- PMID: 34451906
- PMCID: PMC8402219
- DOI: 10.3390/ph14080809
A High-Content Screen for the Identification of Plant Extracts with Insulin Secretion-Modulating Activity
Abstract
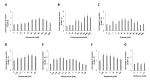
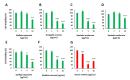
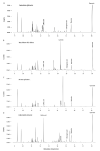
Bioactive plant compounds and extracts are of special interest for the development of pharmaceuticals. Here, we describe the screening of more than 1100 aqueous plant extracts and synthetic reference compounds for their ability to stimulate or inhibit insulin secretion. To quantify insulin secretion in living MIN6 β cells, an insulin-Gaussia luciferase (Ins-GLuc) biosensor was used. Positive hits included extracts from Quillaja saponaria, Anagallis arvensis, Sapindus mukorossi, Gleditsia sinensis and Albizia julibrissin, which were identified as insulin secretion stimulators, whereas extracts of Acacia catechu, Myrtus communis, Actaea spicata L., Vaccinium vitis-idaea and Calendula officinalis were found to exhibit insulin secretion inhibitory properties. Gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) were used to characterize several bioactive compounds in the selected plant extracts, and these bioactives were retested for their insulin-modulating properties. Overall, we identified several plant extracts and some of their bioactive compounds that may be used to manipulate pancreatic insulin secretion.
Keywords: GC-MS; LC-MS; Western blotting; bioactives; diabetes; insulin; luciferase; natural compounds; natural plant extracts; screening; β cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures











Similar articles
-
Insulin promoter-driven Gaussia luciferase-based insulin secretion biosensor assay for discovery of β-cell glucose-sensing pathways.ACS Sens. 2016 Oct 28;1(10):1208-1212. doi: 10.1021/acssensors.6b00433. Epub 2016 Oct 12. ACS Sens. 2016. PMID: 27819058 Free PMC article.
-
Therapeutic propensities, phytochemical composition, and toxicological evaluation of Anagallis arvensis (L.): A wild edible medicinal food plant.Food Res Int. 2020 Nov;137:109651. doi: 10.1016/j.foodres.2020.109651. Epub 2020 Sep 9. Food Res Int. 2020. PMID: 33233230
-
Chemical composition of Acacia farnesiana (L) wild fruits and its activity against Mycobacterium tuberculosis and dysentery bacteria.J Ethnopharmacol. 2019 Feb 10;230:74-80. doi: 10.1016/j.jep.2018.10.031. Epub 2018 Oct 25. J Ethnopharmacol. 2019. PMID: 30367988
-
Role of liquid chromatography-high-resolution mass spectrometry (LC-HR/MS) in clinical toxicology.Clin Toxicol (Phila). 2012 Sep;50(8):733-42. doi: 10.3109/15563650.2012.713108. Epub 2012 Aug 13. Clin Toxicol (Phila). 2012. PMID: 22888997 Review.
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
Cited by
-
Small molecule glucagon release inhibitors with activity in human islets.Front Endocrinol (Lausanne). 2023 Apr 19;14:1114799. doi: 10.3389/fendo.2023.1114799. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37152965 Free PMC article.
-
Small-molecule discovery in the pancreatic beta cell.Curr Opin Chem Biol. 2022 Jun;68:102150. doi: 10.1016/j.cbpa.2022.102150. Epub 2022 Apr 26. Curr Opin Chem Biol. 2022. PMID: 35487100 Free PMC article. Review.
-
Modulatory Effect of Medicinal Plants and Their Active Constituents on ATP-Sensitive Potassium Channels (KATP) in Diabetes.Pharmaceuticals (Basel). 2023 Mar 31;16(4):523. doi: 10.3390/ph16040523. Pharmaceuticals (Basel). 2023. PMID: 37111281 Free PMC article. Review.
-
Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods.Molecules. 2022 Jul 13;27(14):4490. doi: 10.3390/molecules27144490. Molecules. 2022. PMID: 35889361 Free PMC article. Review.
-
Anti-Hyperglycemic Effects of Oils and Extracts Derived from Sea Buckthorn - A Comprehensive Analysis Utilizing In Vitro and In Vivo Models.Mol Nutr Food Res. 2022 Jun;66(12):e2101133. doi: 10.1002/mnfr.202101133. Epub 2022 Apr 27. Mol Nutr Food Res. 2022. PMID: 35426970 Free PMC article.
References
-
- Kakei M., Yoshida M., Dezaki K., Ito K., Yamada H., Funazaki S., Kawakami M., Sugawara H., Yada T. Glucose and GTP-binding protein-coupled receptor cooperatively regulate transient receptor potential-channels to stimulate insulin secretion Review. Endocr. J. 2016;63:867–876. doi: 10.1507/endocrj.EJ16-0262. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

