Protective Efficacy of Novel Oral Biofilm Vaccines against Lactococcus garvieae Infection in Mullet, Mugil cephalus
- PMID: 34451969
- PMCID: PMC8402525
- DOI: 10.3390/vaccines9080844
Protective Efficacy of Novel Oral Biofilm Vaccines against Lactococcus garvieae Infection in Mullet, Mugil cephalus
Abstract
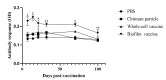
Lactococcus garvieae (L. garvieae) is an important pathogen that causes enormous economic losses in both marine and freshwater aquaculture. At present, antibiotics are the only option for farmers to reduce the losses caused by L. garvieae. However, the usage of antibiotics leads to environmental pollution and the production of drug-resistant strains of bacteria. Therefore, vaccination is preferred as an alternative method to prevent infectious diseases. In this study, we describe an effective approach to the production of an oral biofilm vaccine, using bacteria grown on chitosan particles to form biofilms, and thus providing an inactive pathogen that enhances the immune response in fish. We observed the formation of a biofilm on chitosan particles and administered the novel oral biofilm vaccine to fish. We analyzed the immune responses, including antibody production, phagocytic ability, albumin/globulin ratio and immune-related genes, of vaccinated and control groups of black mullet. Our results show that the phagocytic ability of the biofilm vaccine group was 84%, which is significantly higher than that of the control group, and the antibody production in this group was significantly higher compared with the other group. The mRNA expression levels of immune-related genes (TLR2, IL-1β, TNF-α) were significantly upregulated in the spleen after vaccination. In challenge experiments, the relative percent survival (RPS) was 77% in the biofilm vaccine group, 18% in the whole-cell vaccine group, and 0% in the chitosan particle group at 32 days post-vaccination. In addition, we also found that the relative percent survival (RPS) at 1 day post-vaccination was 74% in the biofilm vaccine group, 42% in the whole-cell vaccine group, and 26% in the chitosan particle group. In both long-term and short-term challenge experiments, the viability of the biofilm vaccine group was significantly higher than that of the whole-cell, chitosan particle and PBS groups. We conclude that based on its protective effect, the L. garvieae biofilm vaccine is better than the whole-cell vaccine when challenged several weeks after vaccination. In addition, the biofilm vaccine also has a greater protective effect than the whole-cell vaccine when challenged immediately after vaccination. Therefore, the biofilm vaccine might represent a novel method for the prevention and treatment of L. garvieae infection.
Keywords: IgM; Lactococcus garvieae; Mugil cephalus; oral biofilm vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Evans J.J., Klesius P.H., Shoemaker C.A. First isolation and characterization of Lactococcus garvieae from Brazilian Nile tilapia, Oreochromis niloticus (L.), and pintado, Pseudoplathystoma corruscans (Spix & Agassiz) J. Fish Dis. 2009;32:943–951. - PubMed
-
- Chen S.C., Liaw L.L., Su H.Y., Ko S.C., Wu C.Y., Chaung H.C., Tsai Y.H., Yang K.L., Chen Y.C., Chen T.H., et al. Lactococcus garvieae, a cause of disease in grey mullet, Mugil cephalus L., in Taiwan. J. Fish Dis. 2002;25:727–732. doi: 10.1046/j.1365-2761.2002.00415.x. - DOI
LinkOut - more resources
Full Text Sources

