Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines
- PMID: 34451973
- PMCID: PMC8402319
- DOI: 10.3390/vaccines9080848
Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines
Abstract
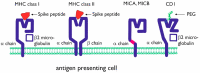
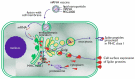
Infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causes Coronavirus Disease 2019 (COVID-19), which has reached pandemic proportions. A number of effective vaccines have been produced, including mRNA vaccines and viral vector vaccines, which are now being implemented on a large scale in order to control the pandemic. The mRNA vaccines are composed of viral Spike S1 protein encoding mRNA incorporated in a lipid nanoparticle and stabilized by polyethylene glycol (PEG). The mRNA vaccines are novel in many respects, including cellular uptake and the intracellular routing, processing, and secretion of the viral protein. Viral vector vaccines have incorporated DNA sequences, encoding the SARS-CoV-2 Spike protein into (attenuated) adenoviruses. The antigen presentation routes in MHC class I and class II, in relation to the induction of virus-neutralizing antibodies and cytotoxic T-lymphocytes, will be reviewed. In rare cases, mRNA vaccines induce unwanted immune mediated side effects. The mRNA-based vaccines may lead to an anaphylactic reaction. This reaction may be triggered by PEG. The intracellular routing of PEG and potential presentation in the context of CD1 will be discussed. Adenovirus vector-based vaccines have been associated with thrombocytopenic thrombosis events. The anti-platelet factor 4 antibodies found in these patients could be generated due to conformational changes of relevant epitopes presented to the immune system.
Keywords: Spike protein; antigen presentation; mRNA vaccine; platelet factor 4; polyethylene glycol; thrombosis; viral vector vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization WHO Coronavirus (Covid-19) Dashboard. [(accessed on 17 June 2021)]; Available online: https://covid19.who.int.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

