Biopharmaceutics 4.0, Advanced Pre-Clinical Development of mRNA-Encoded Monoclonal Antibodies to Immunosuppressed Murine Models
- PMID: 34452015
- PMCID: PMC8402437
- DOI: 10.3390/vaccines9080890
Biopharmaceutics 4.0, Advanced Pre-Clinical Development of mRNA-Encoded Monoclonal Antibodies to Immunosuppressed Murine Models
Abstract
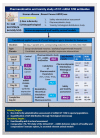
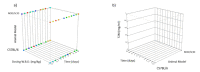
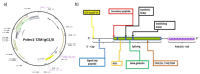
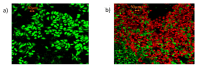
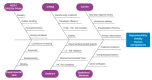
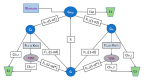
Administration of mRNA against SARS-CoV-2 has demonstrated sufficient efficacy, tolerability and clinical potential to disrupt the vaccination field. A multiple-arm, cohort randomized, mixed blind, placebo-controlled study was designed to investigate the in vivo expression of mRNA antibodies to immunosuppressed murine models to conduct efficacy, safety and bioavailability evaluation. Enabling 4.0 tools we reduced animal sacrifice, while interventions were designed compliant to HARRP and SPIRIT engagement: (a) Randomization, blinding; (b) pharmaceutical grade formulation, monitoring; (c) biochemical and histological analysis; and (d) theoretic, statistical analysis. Risk assessment molded the study orientations, according to the ARRIVE guidelines. The primary target of this protocol is the validation of the research hypothesis that autologous translation of Trastuzumab by in vitro transcribed mRNA-encoded antibodies to immunosuppressed animal models, is non-inferior to classical treatments. The secondary target is the comparative pharmacokinetic assessment of the novel scheme, between immunodeficient and healthy subjects. Herein, the debut clinical protocol, investigating the pharmacokinetic/pharmacodynamic impact of mRNA vaccination to immunodeficient organisms. Our design, contributes novel methodology to guide the preclinical development of RNA antibody modalities by resolving efficacy, tolerability and dose regime adjustment for special populations that are incapable of humoral defense.
Keywords: COVID-19; IVT mRNA; NOD/SCID/J; SARS–CoV-2; Trastuzumab; biopharmaceutics 4.0; immunodeficient; mRNA encoded antibodies; mRNA-vaccines; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: a double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial.Lancet Infect Dis. 2024 Apr;24(4):351-360. doi: 10.1016/S1473-3099(23)00650-3. Epub 2023 Dec 20. Lancet Infect Dis. 2024. PMID: 38141632 Clinical Trial.
-
Oral biopharmaceutics tools: recent progress from partnership through the Pharmaceutical Education and Research with Regulatory Links collaboration.J Pharm Pharmacol. 2021 Mar 8;73(4):437-446. doi: 10.1093/jpp/rgaa055. J Pharm Pharmacol. 2021. PMID: 33793836 Review.
Cited by
-
COMPARING vaccine manufacturing technologies recombinant DNA vs in vitro transcribed (IVT) mRNA.Sci Rep. 2024 Sep 18;14(1):21742. doi: 10.1038/s41598-024-67797-x. Sci Rep. 2024. PMID: 39289418 Free PMC article.
-
mRNA Therapeutic Modalities Design, Formulation and Manufacturing under Pharma 4.0 Principles.Biomedicines. 2021 Dec 27;10(1):50. doi: 10.3390/biomedicines10010050. Biomedicines. 2021. PMID: 35052730 Free PMC article. Review.
-
Advancements and challenges in mRNA and ribonucleoprotein-based therapies: From delivery systems to clinical applications.Mol Ther Nucleic Acids. 2024 Aug 19;35(3):102313. doi: 10.1016/j.omtn.2024.102313. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39281702 Free PMC article. Review.
-
Pharma 4.0 Continuous mRNA Drug Products Manufacturing.Pharmaceutics. 2021 Aug 31;13(9):1371. doi: 10.3390/pharmaceutics13091371. Pharmaceutics. 2021. PMID: 34575447 Free PMC article.
References
-
- Merlo L.M.F., Mandik-Nayak L. Adaptive Immunity: B Cells and Antibodies. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2013.
LinkOut - more resources
Full Text Sources
Miscellaneous

