Efficacy of Two Chlamydia abortus Subcellular Vaccines in a Pregnant Ewe Challenge Model for Ovine Enzootic Abortion
- PMID: 34452023
- PMCID: PMC8402522
- DOI: 10.3390/vaccines9080898
Efficacy of Two Chlamydia abortus Subcellular Vaccines in a Pregnant Ewe Challenge Model for Ovine Enzootic Abortion
Abstract
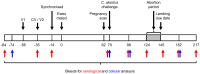
Chlamydia abortus, the aetiological agent of enzootic abortion of ewes, is a major cause of reproductive loss in small ruminants worldwide, accounting for significant economic losses to the farming industry. Disease can be managed through the use of commercial inactivated or live whole organism-based vaccines, although both have limitations particularly in terms of efficacy, safety and disease-associated outbreaks. Here we report a comparison of two experimental vaccines (chlamydial outer membrane complex (COMC) and octyl glucoside (OG)-COMC) based on detergent extracted outer membrane preparations of C. abortus and delivered as prime-boost immunisations, with the commercial live vaccine Cevac® Chlamydia in a pregnant sheep challenge model. No abortions occurred in either experimental vaccine group, while a single abortion occurred in the commercial vaccine group. Bacterial shedding, as a measure of potential risk of transmission of infection to naïve animals, was lowest in the COMC vaccinated group, with reductions of 87.5%, 86.4% and 74% observed for the COMC, OG-COMC and live commercial vaccine groups, respectively, compared to the unvaccinated challenge control group. The results show that the COMC vaccine performed the best and is a safer efficacious alternative to the commercial vaccines. However, to improve commercial viability, future studies should optimise the antigen dose and number of inoculations required.
Keywords: Chlamydia abortus; cytokine analysis; enzootic abortion of ewes; quantitative real-time polymerase chain reaction (PCR); serological analysis; vaccine development; vaccine efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Protective Efficacy of Decreasing Antigen Doses of a Chlamydia abortus Subcellular Vaccine Against Ovine Enzootic Abortion in a Pregnant Sheep Challenge Model.Vaccines (Basel). 2025 Jan 18;13(1):89. doi: 10.3390/vaccines13010089. Vaccines (Basel). 2025. PMID: 39852869 Free PMC article.
-
Evaluation of the Protective Efficacy of Different Doses of a Chlamydia abortus Subcellular Vaccine in a Pregnant Sheep Challenge Model for Ovine Enzootic Abortion.Animals (Basel). 2024 Oct 17;14(20):3004. doi: 10.3390/ani14203004. Animals (Basel). 2024. PMID: 39457934 Free PMC article.
-
Evaluation of the Efficacy of a New Commercially Available Inactivated Vaccine Against Ovine Enzootic Abortion.Front Vet Sci. 2020 Sep 4;7:593. doi: 10.3389/fvets.2020.00593. eCollection 2020. Front Vet Sci. 2020. PMID: 33102549 Free PMC article.
-
New challenges for vaccination to prevent chlamydial abortion in sheep.Comp Immunol Microbiol Infect Dis. 2012 May;35(3):271-6. doi: 10.1016/j.cimid.2011.12.001. Epub 2011 Dec 30. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22209689 Review.
-
Protective adaptive immunity to Chlamydophila abortus infection and control of ovine enzootic abortion (OEA).Vet Microbiol. 2009 Mar 16;135(1-2):112-21. doi: 10.1016/j.vetmic.2008.09.030. Epub 2008 Sep 13. Vet Microbiol. 2009. PMID: 18952388 Review.
Cited by
-
Surface Display of Cholera Toxin B Subunit Recombinant Escherichia coli Ghosts Further Enhances Resistance to Chlamydia abortus Infection in Mice.Microorganisms. 2024 Aug 13;12(8):1656. doi: 10.3390/microorganisms12081656. Microorganisms. 2024. PMID: 39203498 Free PMC article.
-
Seroprevalence and Risk Factors Associated with Chlamydia abortus Infection in Sheep and Goats in North-Western Italy.Animals (Basel). 2024 Jan 17;14(2):291. doi: 10.3390/ani14020291. Animals (Basel). 2024. PMID: 38254460 Free PMC article.
-
Chlamydiosis in Animals.Animals (Basel). 2024 Oct 30;14(21):3130. doi: 10.3390/ani14213130. Animals (Basel). 2024. PMID: 39518853 Free PMC article. Review.
-
Inactivated Flagellin-Containing Vaccine Efficacy against Ovine Enzootic Abortion.Pathogens. 2024 Mar 24;13(4):277. doi: 10.3390/pathogens13040277. Pathogens. 2024. PMID: 38668231 Free PMC article.
-
Coxiella burnetii and Co-Infections with Other Major Pathogens Causing Abortion in Small Ruminant Flocks in the Iberian Peninsula.Animals (Basel). 2022 Dec 7;12(24):3454. doi: 10.3390/ani12243454. Animals (Basel). 2022. PMID: 36552374 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

