Pheophorbide A and Paclitaxel Bioresponsive Nanoparticles as Double-Punch Platform for Cancer Therapy
- PMID: 34452091
- PMCID: PMC8399365
- DOI: 10.3390/pharmaceutics13081130
Pheophorbide A and Paclitaxel Bioresponsive Nanoparticles as Double-Punch Platform for Cancer Therapy
Abstract
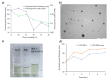
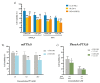
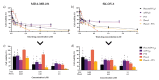
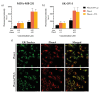
Cancer therapy is still a challenging issue. To address this, the combination of anticancer drugs with other therapeutic modalities, such as light-triggered therapies, has emerged as a promising approach, primarily when both active ingredients are provided within a single nanosystem. Herein, we describe the unprecedented preparation of tumor microenvironment (TME) responsive nanoparticles exclusively composed of a paclitaxel (PTX) prodrug and the photosensitizer pheophorbide A (PheoA), e.g., PheoA≅PTX2S. This system aimed to achieve both the TME-triggered and controlled release of PTX and the synergistic/additive effect by PheoA-mediated photodynamic therapy. PheoA≅PTX2S were produced in a simple one-pot process, exhibiting excellent reproducibility, stability, and the ability to load up to 100% PTX and 40% of PheoA. Exposure of PheoA≅PTX2S nanoparticles to TME-mimicked environment provided fast disassembly compared to normal conditions, leading to PTX and PheoA release and consequently elevated cytotoxicity. Our data indicate that PheoA incorporation into nanoparticles prevents its aggregation, thus providing a greater extent of ROS and singlet oxygen production. Importantly, in SK-OV-3 cells, PheoA≅PTX2S allowed a 30-fold PTX dose reduction and a 3-fold dose reduction of PheoA. Our data confirm that prodrug-based nanocarriers represent valuable and sustainable drug delivery systems, possibly reducing toxicity and expediting preclinical and clinical translation.
Keywords: nanoparticles; paclitaxel; pheophorbide A; photodynamic therapy; prodrug; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


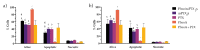
References
Grants and funding
LinkOut - more resources
Full Text Sources

