Formulation and Efficacy of Catalase-Loaded Nanoparticles for the Treatment of Neonatal Hypoxic-Ischemic Encephalopathy
- PMID: 34452092
- PMCID: PMC8400001
- DOI: 10.3390/pharmaceutics13081131
Formulation and Efficacy of Catalase-Loaded Nanoparticles for the Treatment of Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
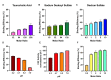
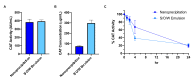
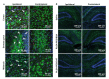
Neonatal hypoxic-ischemic encephalopathy is the leading cause of permanent brain injury in term newborns and currently has no cure. Catalase, an antioxidant enzyme, is a promising therapeutic due to its ability to scavenge toxic reactive oxygen species and improve tissue oxygen status. However, upon in vivo administration, catalase is subject to a short half-life, rapid proteolytic degradation, immunogenicity, and an inability to penetrate the brain. Polymeric nanoparticles can improve pharmacokinetic properties of therapeutic cargo, although encapsulation of large proteins has been challenging. In this paper, we investigated hydrophobic ion pairing as a technique for increasing the hydrophobicity of catalase and driving its subsequent loading into a poly(lactic-co-glycolic acid)-poly(ethylene glycol) (PLGA-PEG) nanoparticle. We found improved formation of catalase-hydrophobic ion complexes with dextran sulfate (DS) compared to sodium dodecyl sulfate (SDS) or taurocholic acid (TA). Molecular dynamics simulations in a model system demonstrated retention of native protein structure after complexation with DS, but not SDS or TA. Using DS-catalase complexes, we developed catalase-loaded PLGA-PEG nanoparticles and evaluated their efficacy in the Vannucci model of unilateral hypoxic-ischemic brain injury in postnatal day 10 rats. Catalase-loaded nanoparticles retained enzymatic activity for at least 24 h in serum-like conditions, distributed through injured brain tissue, and delivered a significant neuroprotective effect compared to saline and blank nanoparticle controls. These results encourage further investigation of catalase and PLGA-PEG nanoparticle-mediated drug delivery for the treatment of neonatal brain injury.
Keywords: catalase; hydrophobic-ion pairing; hypoxia-ischemia; molecular dynamics; nanomedicine; neonatal.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Shankaran S., Laptook A.R., Pappas A., McDonald S.A., Das A., Tyson J.E., Poindexter B.B., Schibler K., Bell E.F., Heyne R.J., et al. Effect of Depth and Duration of Cooling on Death or Disability at Age 18 Months Among Neonates with Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA. 2017;318:57–67. doi: 10.1001/jama.2017.7218. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

